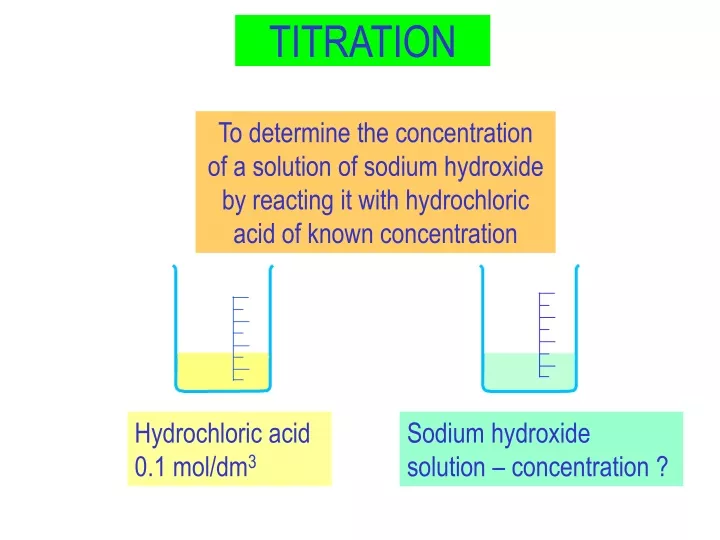
Titration Definition Slideshare . The power point presentation can be downloaded from this. Lecture 16 deals with solutions. In a titration a solution of accurately known concentration is gradually added to another. A titration is a technique for finding an unknown concentration of one chemical from the known. Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume of another solution of unknown concentration (called. An indicator is used to determine when the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base.
from www.slideserve.com
An indicator is used to determine when the. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. In a titration a solution of accurately known concentration is gradually added to another. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. Lecture 16 deals with solutions. A titration is a technique for finding an unknown concentration of one chemical from the known. The power point presentation can be downloaded from this. Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume of another solution of unknown concentration (called.
PPT TITRATION PowerPoint Presentation, free download ID9336460
Titration Definition Slideshare Lecture 16 deals with solutions. Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume of another solution of unknown concentration (called. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. Lecture 16 deals with solutions. In a titration a solution of accurately known concentration is gradually added to another. The power point presentation can be downloaded from this. An indicator is used to determine when the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. A titration is a technique for finding an unknown concentration of one chemical from the known.
From www.youtube.com
Chemistry & Biology Define the Term Titration YouTube Titration Definition Slideshare An indicator is used to determine when the. In a titration a solution of accurately known concentration is gradually added to another. A titration is a technique for finding an unknown concentration of one chemical from the known. The power point presentation can be downloaded from this. Titration is the slow addition of one solution of a known concentration (called. Titration Definition Slideshare.
From studyespacioec4o.z22.web.core.windows.net
How To Do Titrations In Chemistry Titration Definition Slideshare Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume of another solution of unknown concentration (called. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. An indicator is used. Titration Definition Slideshare.
From gamesmartz.com
Titration Definition & Image GameSmartz Titration Definition Slideshare Lecture 16 deals with solutions. In a titration a solution of accurately known concentration is gradually added to another. Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume of another solution of unknown concentration (called. The power point presentation can be downloaded from this. A titration is a. Titration Definition Slideshare.
From www.slideserve.com
PPT Chem. 31 2/3 Lecture PowerPoint Presentation, free download Titration Definition Slideshare An indicator is used to determine when the. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. The power point presentation can be downloaded from this. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. Titration Definition Slideshare.
From www.slideserve.com
PPT Intro to Titrations PowerPoint Presentation, free download ID Titration Definition Slideshare A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. The power point presentation can be downloaded from this. A titration is a technique for finding an unknown concentration of one chemical from the known. Titration is the slow addition of one solution of a known concentration. Titration Definition Slideshare.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5970774 Titration Definition Slideshare In a titration a solution of accurately known concentration is gradually added to another. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. An indicator is used to determine when the. A titration is a technique for finding an unknown concentration. Titration Definition Slideshare.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID9336460 Titration Definition Slideshare A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. Lecture 16 deals with solutions. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. Titration is the slow. Titration Definition Slideshare.
From scienceinfo.com
Titration Definition, 4 Types, Procedure Titration Definition Slideshare A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. Lecture 16 deals with solutions. In a titration a solution of accurately known concentration is gradually added to another. A titration is a technique for finding an unknown concentration of one chemical from the known. Titration is. Titration Definition Slideshare.
From www.youtube.com
Titration Definition, steps and formula YouTube Titration Definition Slideshare An indicator is used to determine when the. Lecture 16 deals with solutions. Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume of another solution of unknown concentration (called. In a titration a solution of accurately known concentration is gradually added to another. A titration is a technique. Titration Definition Slideshare.
From www.youtube.com
titration part1 definition YouTube Titration Definition Slideshare Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. A titration is a technique for finding an unknown concentration of one chemical from the known. The power point presentation can be downloaded from this. A lab practice used to determine the. Titration Definition Slideshare.
From studylib.net
Titration Titration Definition Slideshare In a titration a solution of accurately known concentration is gradually added to another. The power point presentation can be downloaded from this. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. An indicator is used to determine when the. A titration is a technique for. Titration Definition Slideshare.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Definition Slideshare An indicator is used to determine when the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. In a titration a solution of accurately known concentration is gradually added to another. Lecture 16 deals with solutions. The power point presentation can. Titration Definition Slideshare.
From www.tes.com
Titrations Required Practical Sheet Teaching Resources Titration Definition Slideshare A titration is a technique for finding an unknown concentration of one chemical from the known. An indicator is used to determine when the. In a titration a solution of accurately known concentration is gradually added to another. The power point presentation can be downloaded from this. Lecture 16 deals with solutions. Titration is the slow addition of one solution. Titration Definition Slideshare.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Definition Slideshare In a titration a solution of accurately known concentration is gradually added to another. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to. Titration Definition Slideshare.
From www.aiophotoz.com
Titration Vector Illustration Labeled Educational Chemistry Process Titration Definition Slideshare A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume of another solution of unknown concentration (called. Lecture 16 deals with solutions. In a titration a. Titration Definition Slideshare.
From www.slideserve.com
PPT Ch 6 Good Titrations PowerPoint Presentation ID3254207 Titration Definition Slideshare The power point presentation can be downloaded from this. A titration is a technique for finding an unknown concentration of one chemical from the known. Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume of another solution of unknown concentration (called. An indicator is used to determine when. Titration Definition Slideshare.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Definition Slideshare Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. An indicator is used to determine when the. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. In. Titration Definition Slideshare.
From www.slideserve.com
PPT 09 Titrations & their CALCULATIONS PowerPoint Presentation ID Titration Definition Slideshare An indicator is used to determine when the. A titration is a technique for finding an unknown concentration of one chemical from the known. The power point presentation can be downloaded from this. Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume of another solution of unknown concentration. Titration Definition Slideshare.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration Definition Slideshare Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume of another solution of unknown concentration (called. An indicator is used to determine when the. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. The. Titration Definition Slideshare.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID4319437 Titration Definition Slideshare A titration is a technique for finding an unknown concentration of one chemical from the known. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. An indicator is used to determine when the. The power point presentation can be downloaded from. Titration Definition Slideshare.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Definition Slideshare The power point presentation can be downloaded from this. In a titration a solution of accurately known concentration is gradually added to another. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. An indicator is used to determine when the. Titration is the slow addition of. Titration Definition Slideshare.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Definition Slideshare Lecture 16 deals with solutions. The power point presentation can be downloaded from this. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. In a titration a solution of accurately known concentration is gradually added to another. A lab practice used. Titration Definition Slideshare.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Definition Slideshare Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. An indicator is used to determine when the. In a titration a solution of accurately known concentration is gradually added to another. The power point presentation can be downloaded from this. Titration. Titration Definition Slideshare.
From acidsandbasesandmore.weebly.com
Titrations CHEMISTRY 101 Titration Definition Slideshare A titration is a technique for finding an unknown concentration of one chemical from the known. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. The power point presentation can be downloaded from this. In a titration a solution of accurately known concentration is gradually added. Titration Definition Slideshare.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Definition Slideshare Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume of another solution of unknown concentration (called. The power point presentation can be downloaded from this. Lecture 16 deals with solutions. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with. Titration Definition Slideshare.
From www.slideshare.net
Titration or titrimetry Titration Definition Slideshare A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. Lecture 16 deals with solutions. A titration is a technique for finding an unknown concentration of one chemical from the known. The power point presentation can be downloaded from this. In a titration a solution of accurately. Titration Definition Slideshare.
From www.slideserve.com
PPT Titrations. . . PowerPoint Presentation, free download ID5607639 Titration Definition Slideshare Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume of another solution of unknown concentration (called. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. A titration is a technique for finding an unknown. Titration Definition Slideshare.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Definition Slideshare A titration is a technique for finding an unknown concentration of one chemical from the known. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume. Titration Definition Slideshare.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and Titration Definition Slideshare Lecture 16 deals with solutions. In a titration a solution of accurately known concentration is gradually added to another. An indicator is used to determine when the. The power point presentation can be downloaded from this. A titration is a technique for finding an unknown concentration of one chemical from the known. Titration is the slow addition of one solution. Titration Definition Slideshare.
From www.showme.com
Topic Titrations ShowMe Online Learning Titration Definition Slideshare The power point presentation can be downloaded from this. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. An indicator is used to determine when the. In a titration a solution of accurately known concentration is gradually added to another. Titration is the slow addition of. Titration Definition Slideshare.
From www.slideshare.net
Titration Aparatus Titration Definition Slideshare An indicator is used to determine when the. In a titration a solution of accurately known concentration is gradually added to another. The power point presentation can be downloaded from this. Lecture 16 deals with solutions. Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a known volume of another solution. Titration Definition Slideshare.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Titration Definition Slideshare Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. Lecture 16 deals with solutions. A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. The power point presentation. Titration Definition Slideshare.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2145156 Titration Definition Slideshare Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. Lecture 16 deals with solutions. An indicator is used to determine when the. Titration is the slow addition of one solution of a known concentration (called a titrant or titrator) to a. Titration Definition Slideshare.
From www.slideserve.com
PPT Precipitation titration PowerPoint Presentation, free download Titration Definition Slideshare In a titration a solution of accurately known concentration is gradually added to another. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown concentration until the reaction. A titration is a technique for finding an unknown concentration of one chemical from the known. A lab. Titration Definition Slideshare.
From www.osmosis.org
Introduction to titrations Vídeo & Anatomía Osmosis Titration Definition Slideshare A lab practice used to determine the concentration of an unknown acid or base by neutralizing it with a known acid or base. A titration is a technique for finding an unknown concentration of one chemical from the known. An indicator is used to determine when the. Lecture 16 deals with solutions. In a titration a solution of accurately known. Titration Definition Slideshare.