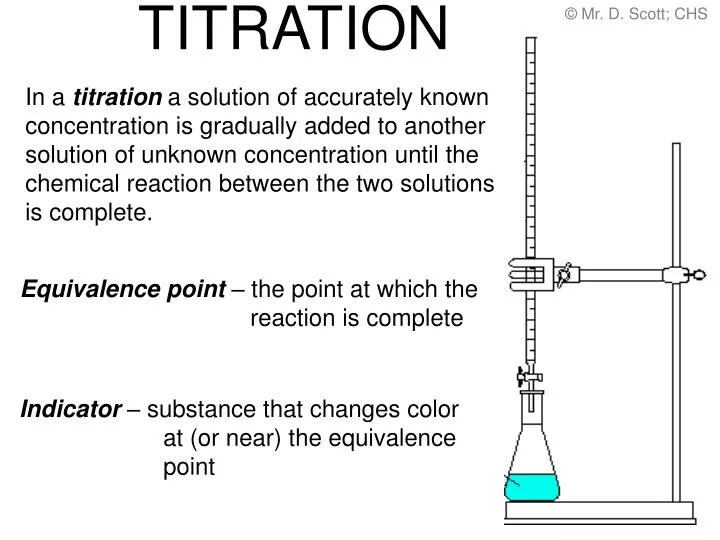
Titration Lab Purpose . Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Perform and interpret titration calculations. Find the molarity of a solution with an unknown concentration. Titration is useful for many applications such as: Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is an inflection in the slope of the curve. A chemical reaction is set up between a known volume of a solution of.
from www.slideserve.com
A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is an inflection in the slope of the curve. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration is useful for many applications such as: The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Perform and interpret titration calculations. A chemical reaction is set up between a known volume of a solution of. Find the molarity of a solution with an unknown concentration. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown.
PPT TITRATION PowerPoint Presentation, free download ID1459481
Titration Lab Purpose Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. A chemical reaction is set up between a known volume of a solution of. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is an inflection in the slope of the curve. Titration is useful for many applications such as: A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Perform and interpret titration calculations. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Find the molarity of a solution with an unknown concentration.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Lab Purpose A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. Perform and interpret titration calculations. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar. Titration Lab Purpose.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Lab Purpose Find the molarity of a solution with an unknown concentration. A chemical reaction is set up between a known volume of a solution of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is useful for many applications such as: In a ph titration. Titration Lab Purpose.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Lab Purpose Perform and interpret titration calculations. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is an inflection in the slope of the curve. The purpose of any titration is to determine the amount of analyte present by reacting away every last. Titration Lab Purpose.
From www.sliderbase.com
Titration Titration Lab Purpose Find the molarity of a solution with an unknown concentration. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is an inflection in the slope of the curve. The purpose of any titration is to determine the amount of analyte present. Titration Lab Purpose.
From ideplugin.com
AcidBase Titration Lab — DataClassroom Lab report AcidBase Titration Lab Purpose Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Find the molarity of a solution with an unknown concentration. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. Perform and interpret titration calculations. Titration is useful for many applications. Titration Lab Purpose.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Lab Purpose A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. A chemical reaction is set up between a known volume of a solution of. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where. Titration Lab Purpose.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Lab Purpose Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Find the molarity of a solution with an unknown concentration. A chemical reaction is set up between a known volume of a solution of. The purpose of any titration is to determine the amount of analyte. Titration Lab Purpose.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Titration Lab Purpose The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A chemical reaction is set up between a known volume of a solution of.. Titration Lab Purpose.
From www.studocu.com
Acid Base Titration Lab Report Purpose In part one of this lab Titration Lab Purpose Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Perform and interpret titration calculations. Titration is useful for many applications such as: Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. The purpose of any. Titration Lab Purpose.
From hubpages.com
Different Methods of Measuring Drug Potency, Concentration, Efficacy Titration Lab Purpose Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration is useful for many applications such as: Find the molarity of a solution with an unknown concentration. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as. Titration Lab Purpose.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Lab Purpose The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a procedure used in chemistry in order to determine the molarity of. Titration Lab Purpose.
From www.youtube.com
Lab Demonstration Acid Base Titration. YouTube Titration Lab Purpose Find the molarity of a solution with an unknown concentration. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is an inflection in the slope of the curve. Perform and interpret titration calculations. A titration is a technique where a solution. Titration Lab Purpose.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Lab Purpose A chemical reaction is set up between a known volume of a solution of. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is an inflection in the slope of the curve. A titration is a laboratory technique used to precisely. Titration Lab Purpose.
From www.vrogue.co
Lab Acid Base Titration Purpose Introductionneimanwik vrogue.co Titration Lab Purpose A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. Perform and interpret titration calculations. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Titration is a procedure used in chemistry in order to determine the molarity. Titration Lab Purpose.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Titration Lab Purpose In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is an inflection in the slope of the curve. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration Lab Purpose.
From scienceready.com.au
Titration Standard Solution, Washing, Setup HSC Chemistry Science Titration Lab Purpose Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Perform and interpret titration calculations. A chemical reaction is set up between a known volume of a solution of. The purpose of any titration is to determine the amount of analyte present by reacting away every. Titration Lab Purpose.
From www.learnsci.com
LearnSci LabSim Titration End Point Titration Lab Purpose A chemical reaction is set up between a known volume of a solution of. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown.. Titration Lab Purpose.
From aylinmeowstuart.blogspot.com
Acid Base Titration Experiment Report Titration Lab Purpose A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In a ph titration you measure the ph as a function of the volume of titrant added. Titration Lab Purpose.
From www.youtube.com
Unit 4.6 Introduction to Titration YouTube Titration Lab Purpose In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is an inflection in the slope of the curve. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration is useful for. Titration Lab Purpose.
From haileeqohicks.blogspot.com
Acid Base Titration Experiment Report HaileeqoHicks Titration Lab Purpose Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A chemical reaction is set up between a known volume of a solution of. Titration is useful for many applications such as: A titration is a technique where a solution of known concentration is used to. Titration Lab Purpose.
From studiousguy.com
Titration Uses in Real Life StudiousGuy Titration Lab Purpose Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance.. Titration Lab Purpose.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Lab Purpose In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is an inflection in the slope of the curve. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. Find the molarity. Titration Lab Purpose.
From mssmithsechs.weebly.com
Acids, Bases, Solutions, and Titrations MS. SMITH'S CLASS Titration Lab Purpose A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. In a ph titration you measure the ph as a function of the volume of titrant added and. Titration Lab Purpose.
From www.visionlearning.com
Acids and Bases I Math in Science Visionlearning Titration Lab Purpose A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration is useful for many applications such as: A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. Find the molarity of a solution with an unknown concentration. Titration is a. Titration Lab Purpose.
From betterlesson.com
Eleventh grade Lesson Titration Lab BetterLesson Titration Lab Purpose Titration is useful for many applications such as: Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A chemical reaction is set up between a known. Titration Lab Purpose.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Titration Lab Purpose Titration is useful for many applications such as: A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is an inflection in. Titration Lab Purpose.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Lab Purpose Perform and interpret titration calculations. A chemical reaction is set up between a known volume of a solution of. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Find the molarity of a solution with an unknown concentration. Titration is a procedure used in chemistry in order to determine the. Titration Lab Purpose.
From utamatani.com
AcidBase Titration Lab — DataClassroom ACIDBASE TITRATION Purpose Titration Lab Purpose A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. Find the molarity of a solution with an unknown concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Perform and interpret titration calculations. A. Titration Lab Purpose.
From fineartamerica.com
Equipment Used In A Titration Photograph by Martyn F. Chillmaid/science Titration Lab Purpose A chemical reaction is set up between a known volume of a solution of. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Titration is useful for many applications such as: In a ph titration you measure the ph as a function of the volume of titrant. Titration Lab Purpose.
From www.studocu.com
Titration Lab Report About the lab tiration Titration Lab Report Titration Lab Purpose Perform and interpret titration calculations. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. A titration is a technique where a solution of known concentration is used to determine the concentration. Titration Lab Purpose.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Lab Purpose A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. Titration is useful for many applications such as: The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Perform and interpret titration calculations. Titration is the slow addition. Titration Lab Purpose.
From mungfali.com
Acid Base Titration Procedure Titration Lab Purpose In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is an inflection in the slope of the curve. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. A titration is. Titration Lab Purpose.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Lab Purpose Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Find the molarity of a solution with an unknown concentration. Perform and interpret titration calculations. Titration is useful for many applications such as: A chemical reaction is set up between a known volume of a solution of. In a ph titration. Titration Lab Purpose.
From www.studocu.com
Lab Manual Acid Base Titration Curves AB ACIDBASE TITRATION Titration Lab Purpose A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A chemical reaction is set up between a known volume of a solution of. A titration is a technique where a solution. Titration Lab Purpose.
From vdocuments.mx
ACIDBASE TITRATION LAB to Deft Studios! · PDF file · 200703 Titration Lab Purpose A chemical reaction is set up between a known volume of a solution of. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. Perform and interpret titration calculations. Titration is useful for many applications such as: Titration is the slow addition of one solution of a known concentration (called. Titration Lab Purpose.