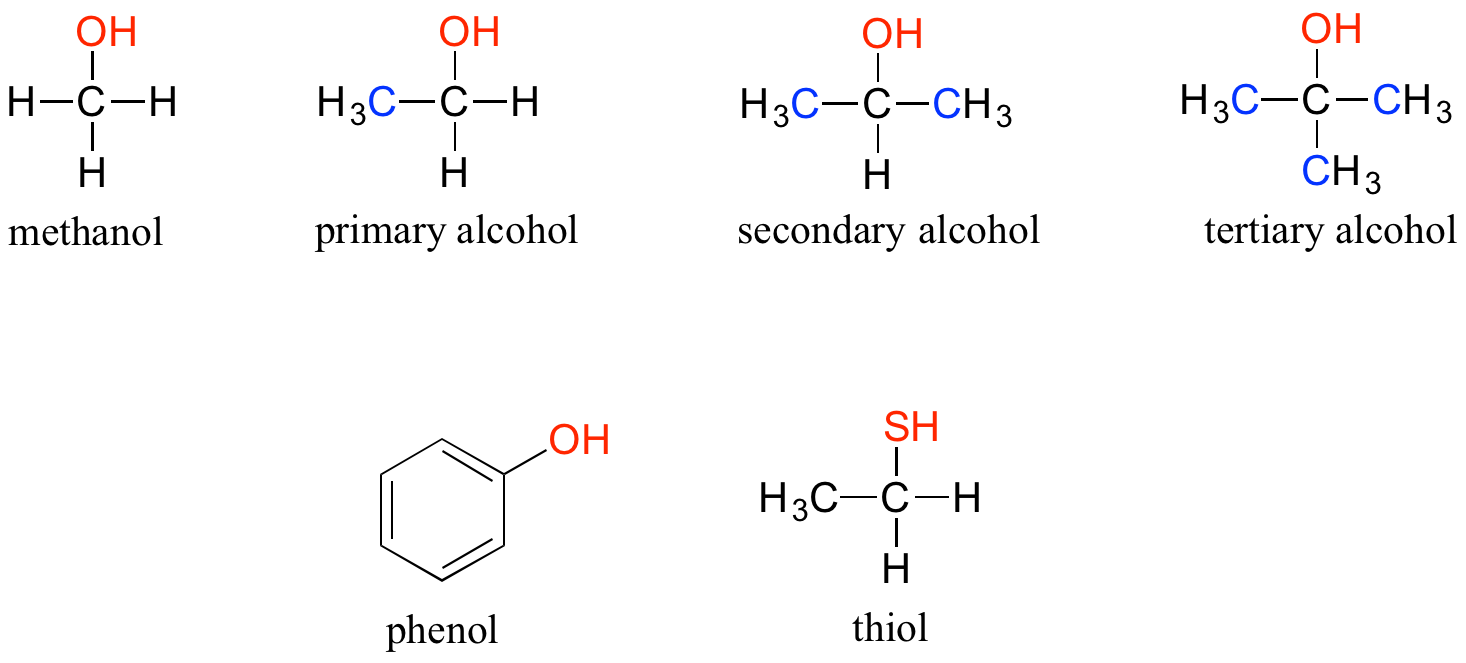
Are Phenols Alcohols . Discuss the factors that are believed to determine the acidity of alcohols and phenols. Phenols have an oh group and are somewhat soluble in water. Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). List a given series of alcohols or phenols in order of increasing or decreasing acidity. They are therefore soluble in dilute aqueous naoh and. Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. Phenols have an oh group attached directly to an aromatic ring. Alcohols are mostly colourless, and they usually exist in the liquid state. Phenols are colourless solids that usually exist as crystals at stp. Alcohols show no impact or reaction during tests as they are mostly neutral. The hydroxyl group of alcohols and phenols is responsible for an interesting.
from chem.libretexts.org
Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). Alcohols are mostly colourless, and they usually exist in the liquid state. Phenols have an oh group attached directly to an aromatic ring. Discuss the factors that are believed to determine the acidity of alcohols and phenols. They are therefore soluble in dilute aqueous naoh and. Alcohols show no impact or reaction during tests as they are mostly neutral. List a given series of alcohols or phenols in order of increasing or decreasing acidity. Phenols are colourless solids that usually exist as crystals at stp. Phenols have an oh group and are somewhat soluble in water. Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous.
1.3 Functional groups and organic nomenclature Chemistry LibreTexts
Are Phenols Alcohols They are therefore soluble in dilute aqueous naoh and. Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). They are therefore soluble in dilute aqueous naoh and. Phenols have an oh group and are somewhat soluble in water. Phenols are colourless solids that usually exist as crystals at stp. Discuss the factors that are believed to determine the acidity of alcohols and phenols. Phenols have an oh group attached directly to an aromatic ring. List a given series of alcohols or phenols in order of increasing or decreasing acidity. The hydroxyl group of alcohols and phenols is responsible for an interesting. Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. Alcohols show no impact or reaction during tests as they are mostly neutral. Alcohols are mostly colourless, and they usually exist in the liquid state.
From mungfali.com
Phenol Lewis Structure Are Phenols Alcohols List a given series of alcohols or phenols in order of increasing or decreasing acidity. Phenols are colourless solids that usually exist as crystals at stp. They are therefore soluble in dilute aqueous naoh and. The hydroxyl group of alcohols and phenols is responsible for an interesting. Alcohols are mostly colourless, and they usually exist in the liquid state. Both. Are Phenols Alcohols.
From www.slideserve.com
PPT Structures of Alcohols, Phenols, Thiols and Ethers PowerPoint Are Phenols Alcohols The hydroxyl group of alcohols and phenols is responsible for an interesting. Alcohols are mostly colourless, and they usually exist in the liquid state. They are therefore soluble in dilute aqueous naoh and. Phenols have an oh group and are somewhat soluble in water. List a given series of alcohols or phenols in order of increasing or decreasing acidity. Phenols. Are Phenols Alcohols.
From www.cleariitmedical.com
Alcohols, Phenols and Ethers Chemistry Notes for IITJEE/NEET Are Phenols Alcohols Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. Discuss the factors that are believed to determine the acidity of alcohols and phenols. Alcohols show no impact or reaction during tests as they are mostly neutral. Phenols have an oh group attached directly to an aromatic ring. They are therefore soluble in dilute aqueous naoh and.. Are Phenols Alcohols.
From byjus.com
What is the order of acidity of alcohols and phenols? Explain both in Are Phenols Alcohols The hydroxyl group of alcohols and phenols is responsible for an interesting. Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). Alcohols are mostly colourless, and they usually exist in the liquid state. Phenols are colourless solids that usually exist as crystals at stp. Alcohols show no impact or reaction during tests as they are mostly neutral.. Are Phenols Alcohols.
From slideplayer.com
Chapter 17 Alcohols and Phenols ppt download Are Phenols Alcohols Alcohols show no impact or reaction during tests as they are mostly neutral. They are therefore soluble in dilute aqueous naoh and. Discuss the factors that are believed to determine the acidity of alcohols and phenols. Phenols have an oh group and are somewhat soluble in water. The hydroxyl group of alcohols and phenols is responsible for an interesting. Alcohols. Are Phenols Alcohols.
From www.slideserve.com
PPT ORGANIC CHEMISTRY PowerPoint Presentation ID233852 Are Phenols Alcohols Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). They are therefore soluble in dilute aqueous naoh and. Discuss the factors that are believed to determine the acidity of alcohols and phenols. Alcohols show no impact or reaction during tests as they are mostly neutral. The hydroxyl group of alcohols and phenols is responsible for an interesting.. Are Phenols Alcohols.
From leah4sci.com
Phenol Resonance and Acidity Organic Chemistry Video Are Phenols Alcohols Phenols have an oh group and are somewhat soluble in water. Phenols are colourless solids that usually exist as crystals at stp. They are therefore soluble in dilute aqueous naoh and. The hydroxyl group of alcohols and phenols is responsible for an interesting. Discuss the factors that are believed to determine the acidity of alcohols and phenols. List a given. Are Phenols Alcohols.
From www.samareducation.com
Alcohols, Phenols and Ethers Class 12 Notes Chemistry Chapter 11 Are Phenols Alcohols Discuss the factors that are believed to determine the acidity of alcohols and phenols. Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). List a given series of alcohols or phenols in order of increasing or decreasing acidity. Phenols have an oh group attached directly to an aromatic ring. Phenols have an oh group and are somewhat. Are Phenols Alcohols.
From www.doubtnut.com
Carboxylic acids are more acidic than phenols and alchols. Explin Are Phenols Alcohols Discuss the factors that are believed to determine the acidity of alcohols and phenols. They are therefore soluble in dilute aqueous naoh and. Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. List a given series of alcohols or phenols in order of increasing or decreasing acidity. Phenols have an oh group and are somewhat soluble. Are Phenols Alcohols.
From www.researchgate.net
Chemical structure of common phenolic compounds. Download Scientific Are Phenols Alcohols List a given series of alcohols or phenols in order of increasing or decreasing acidity. Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). Phenols have an oh group attached directly to an aromatic ring. Phenols are colourless solids that usually exist as crystals. Are Phenols Alcohols.
From byjus.com
What is the order of acidity of alcohols and phenols? Explain both in Are Phenols Alcohols The hydroxyl group of alcohols and phenols is responsible for an interesting. Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. Alcohols show no impact or reaction during tests as they are mostly neutral. Discuss the factors that are believed to determine the acidity. Are Phenols Alcohols.
From www.slideserve.com
PPT Organic Chemistry II Alcohols, Phenols, Thiols , Ethers, and Are Phenols Alcohols Phenols have an oh group attached directly to an aromatic ring. Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. Phenols have an oh group and are somewhat soluble in water. Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). Discuss the factors that are believed to determine the acidity of alcohols and. Are Phenols Alcohols.
From www.youtube.com
Difference Between Alcohol And Phenol?Class Series YouTube Are Phenols Alcohols Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). Phenols have an oh group and are somewhat soluble in water. The hydroxyl group of alcohols and phenols is responsible for an interesting. Phenols have an oh group attached directly to an aromatic ring. List a given series of alcohols or phenols in order of increasing or decreasing. Are Phenols Alcohols.
From www.pw.live
Difference Between Alcohol And Phenol Are Phenols Alcohols Alcohols show no impact or reaction during tests as they are mostly neutral. Phenols have an oh group attached directly to an aromatic ring. Phenols are colourless solids that usually exist as crystals at stp. Phenols have an oh group and are somewhat soluble in water. Alcohols are mostly colourless, and they usually exist in the liquid state. Phenols are. Are Phenols Alcohols.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID4524523 Are Phenols Alcohols Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). Phenols have an oh group attached directly to an aromatic ring. List a given series of alcohols or phenols in order of increasing or decreasing acidity. Phenols have an oh group and are somewhat soluble in water. Alcohols show no impact or reaction during tests as they are. Are Phenols Alcohols.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID Are Phenols Alcohols Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. They are therefore soluble in dilute aqueous naoh and. Phenols have an oh group attached directly to an aromatic ring. Alcohols are mostly colourless, and they usually exist in the liquid state. Phenols are colourless solids that usually exist as crystals at stp. Phenols are about a. Are Phenols Alcohols.
From byjus.com
Phenols are more acidic than alcohol. Explain why? Are Phenols Alcohols Alcohols are mostly colourless, and they usually exist in the liquid state. Alcohols show no impact or reaction during tests as they are mostly neutral. Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. Phenols are colourless solids that usually exist as crystals at stp. Phenols have an oh group and are somewhat soluble in water.. Are Phenols Alcohols.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID Are Phenols Alcohols Phenols are colourless solids that usually exist as crystals at stp. They are therefore soluble in dilute aqueous naoh and. Alcohols show no impact or reaction during tests as they are mostly neutral. The hydroxyl group of alcohols and phenols is responsible for an interesting. Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). Discuss the factors. Are Phenols Alcohols.
From www.imperialstudy.com
Alcohols Phenols and Ethers Notes for Class 12 Chemistry Imperial Study Are Phenols Alcohols Phenols have an oh group attached directly to an aromatic ring. They are therefore soluble in dilute aqueous naoh and. List a given series of alcohols or phenols in order of increasing or decreasing acidity. Phenols are colourless solids that usually exist as crystals at stp. Alcohols are mostly colourless, and they usually exist in the liquid state. Discuss the. Are Phenols Alcohols.
From pediaa.com
Difference Between Alcohol and Phenol Are Phenols Alcohols Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. Phenols have an oh group and are somewhat soluble in water. Phenols are colourless solids that usually exist as crystals at stp. Discuss the factors that are believed to determine the acidity of alcohols and phenols. Alcohols are mostly colourless, and they usually exist in the liquid. Are Phenols Alcohols.
From collegedunia.com
Nomenclature of Alcohols Phenols and Ethers Rules and Examples Are Phenols Alcohols Phenols have an oh group attached directly to an aromatic ring. Phenols have an oh group and are somewhat soluble in water. They are therefore soluble in dilute aqueous naoh and. Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). List a given series. Are Phenols Alcohols.
From chem.libretexts.org
1.3 Functional groups and organic nomenclature Chemistry LibreTexts Are Phenols Alcohols The hydroxyl group of alcohols and phenols is responsible for an interesting. Alcohols are mostly colourless, and they usually exist in the liquid state. Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. Alcohols show no impact or reaction during tests as they are mostly neutral. Phenols are colourless solids that usually exist as crystals at. Are Phenols Alcohols.
From www.slideshare.net
Alcohols, Phenols, and Ethers Are Phenols Alcohols The hydroxyl group of alcohols and phenols is responsible for an interesting. Phenols are colourless solids that usually exist as crystals at stp. Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. Phenols have an oh group and are somewhat soluble in water. Discuss the factors that are believed to determine the acidity of alcohols and. Are Phenols Alcohols.
From www.slideserve.com
PPT Chapter 17 Alcohols and Phenols PowerPoint Presentation, free Are Phenols Alcohols Alcohols show no impact or reaction during tests as they are mostly neutral. Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). Discuss the factors that are believed to determine the acidity of alcohols and phenols. Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. Alcohols are mostly colourless, and they usually exist. Are Phenols Alcohols.
From dauglas.afphila.com
Nomenclature of Alcohols, Phenols and Ethers Rules and Examples Are Phenols Alcohols List a given series of alcohols or phenols in order of increasing or decreasing acidity. Phenols have an oh group and are somewhat soluble in water. Phenols have an oh group attached directly to an aromatic ring. Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. They are therefore soluble in dilute aqueous naoh and. Phenols. Are Phenols Alcohols.
From www.slideserve.com
PPT Alcohols PowerPoint Presentation, free download ID6508687 Are Phenols Alcohols Alcohols are mostly colourless, and they usually exist in the liquid state. Discuss the factors that are believed to determine the acidity of alcohols and phenols. List a given series of alcohols or phenols in order of increasing or decreasing acidity. Phenols are colourless solids that usually exist as crystals at stp. They are therefore soluble in dilute aqueous naoh. Are Phenols Alcohols.
From pediaa.com
Difference Between Alcohol and Phenol Definition, Structure, Use Are Phenols Alcohols Phenols have an oh group attached directly to an aromatic ring. Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). List a given series of alcohols or phenols in order of increasing or decreasing acidity. Alcohols show no impact or reaction during tests as they are mostly neutral. Discuss the factors that are believed to determine the. Are Phenols Alcohols.
From www.slideserve.com
PPT Classification and Identification of Alcohols and Phenols Are Phenols Alcohols Phenols have an oh group and are somewhat soluble in water. They are therefore soluble in dilute aqueous naoh and. Both alcohols and phenols are widespread in nature, with alcohols being especially ubiquitous. Alcohols are mostly colourless, and they usually exist in the liquid state. Discuss the factors that are believed to determine the acidity of alcohols and phenols. Alcohols. Are Phenols Alcohols.
From studylib.net
Alcohols, Phenols and Ethers Are Phenols Alcohols Phenols have an oh group and are somewhat soluble in water. List a given series of alcohols or phenols in order of increasing or decreasing acidity. Discuss the factors that are believed to determine the acidity of alcohols and phenols. Alcohols are mostly colourless, and they usually exist in the liquid state. They are therefore soluble in dilute aqueous naoh. Are Phenols Alcohols.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID Are Phenols Alcohols Phenols have an oh group and are somewhat soluble in water. Discuss the factors that are believed to determine the acidity of alcohols and phenols. They are therefore soluble in dilute aqueous naoh and. List a given series of alcohols or phenols in order of increasing or decreasing acidity. Both alcohols and phenols are widespread in nature, with alcohols being. Are Phenols Alcohols.
From www.slideserve.com
PPT Chapter 17 Alcohols and Phenols PowerPoint Presentation, free Are Phenols Alcohols Phenols have an oh group attached directly to an aromatic ring. Alcohols are mostly colourless, and they usually exist in the liquid state. The hydroxyl group of alcohols and phenols is responsible for an interesting. Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). Discuss the factors that are believed to determine the acidity of alcohols and. Are Phenols Alcohols.
From www.learninsta.com
Alcohols, Phenols and Ethers Class 12 Notes Chemistry Chapter 11 Are Phenols Alcohols Alcohols are mostly colourless, and they usually exist in the liquid state. They are therefore soluble in dilute aqueous naoh and. List a given series of alcohols or phenols in order of increasing or decreasing acidity. Phenols are colourless solids that usually exist as crystals at stp. Discuss the factors that are believed to determine the acidity of alcohols and. Are Phenols Alcohols.
From www.slideserve.com
PPT Structures of Alcohols, Phenols, Thiols and Ethers PowerPoint Are Phenols Alcohols Alcohols are mostly colourless, and they usually exist in the liquid state. Phenols have an oh group attached directly to an aromatic ring. They are therefore soluble in dilute aqueous naoh and. Phenols are about a million times more acidic than alcohols (table \(\pageindex{1}\)). Phenols are colourless solids that usually exist as crystals at stp. Phenols have an oh group. Are Phenols Alcohols.
From guides.hostos.cuny.edu
Chapter 2 Alcohols, Phenols, Thiols, Ethers CHE 120 Introduction Are Phenols Alcohols The hydroxyl group of alcohols and phenols is responsible for an interesting. They are therefore soluble in dilute aqueous naoh and. List a given series of alcohols or phenols in order of increasing or decreasing acidity. Alcohols show no impact or reaction during tests as they are mostly neutral. Phenols are colourless solids that usually exist as crystals at stp.. Are Phenols Alcohols.
From studylib.net
Alcohols and Phenols Are Phenols Alcohols Phenols are colourless solids that usually exist as crystals at stp. Phenols have an oh group attached directly to an aromatic ring. They are therefore soluble in dilute aqueous naoh and. The hydroxyl group of alcohols and phenols is responsible for an interesting. Alcohols are mostly colourless, and they usually exist in the liquid state. Phenols have an oh group. Are Phenols Alcohols.