Standard Curve In Biochemistry . A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. The stock solution of naoh is. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. The same assay is then performed with samples of unknown concentration. The curve is created from the instrumental. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator:
from www.chegg.com
The stock solution of naoh is. The same assay is then performed with samples of unknown concentration. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. The curve is created from the instrumental.
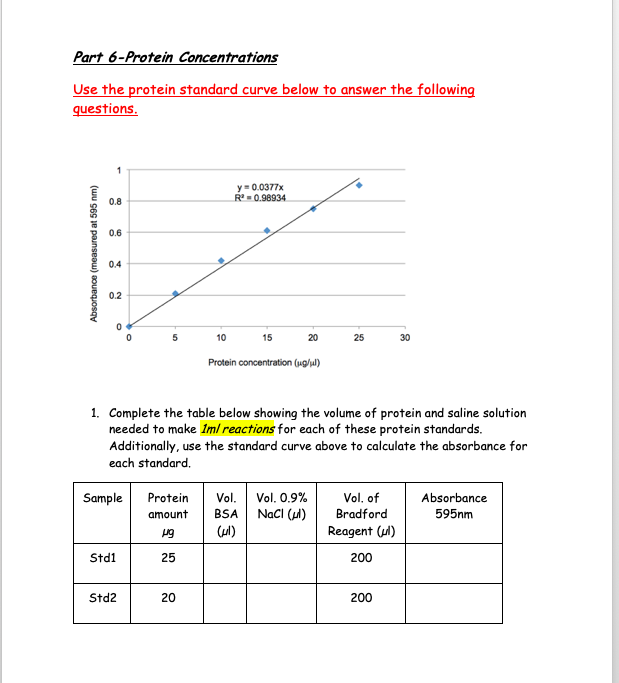
Solved Part 6Protein Concentrations Use the protein
Standard Curve In Biochemistry The same assay is then performed with samples of unknown concentration. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. The same assay is then performed with samples of unknown concentration. The stock solution of naoh is. The curve is created from the instrumental.
From pubs.sciepub.com
The Identification of Amino Acids by Interpretation of Titration Curves Standard Curve In Biochemistry A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. The stock solution of naoh is. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: The same assay is. Standard Curve In Biochemistry.
From www.slideserve.com
PPT Terms Used in Biochemistry & Calculations PowerPoint Presentation Standard Curve In Biochemistry 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. The curve is created from the instrumental. The stock solution of naoh is. The same assay is then performed with samples of unknown concentration. Alcoholic orcinol is also made in advance. Standard Curve In Biochemistry.
From www.youtube.com
Construction of Protein Standard Curve using Folin’s Lowry Method Standard Curve In Biochemistry Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. The curve is created from the instrumental. The same assay is then performed with samples of unknown concentration. The stock solution of naoh is. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. A calibration curve is used to. Standard Curve In Biochemistry.
From www.researchgate.net
Standard curve of the improved lipid assay. Typical standard curve with Standard Curve In Biochemistry 0.60 g orcinol dissolved in 10 ml of 95% ethanol. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. The stock solution of naoh is.. Standard Curve In Biochemistry.
From www.researchgate.net
Standard curves generated from plasmid containing ITS15.8SITS2 rRNA Standard Curve In Biochemistry 0.60 g orcinol dissolved in 10 ml of 95% ethanol. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: 9.7 a focus on understanding standard curves standard curves (sometimes. Standard Curve In Biochemistry.
From www.youtube.com
Preparation of Standard curve for estimation of starch by I2KI method Standard Curve In Biochemistry The stock solution of naoh is. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. Alcoholic. Standard Curve In Biochemistry.
From www.reddit.com
Standard/calibration Curve Issue r/Biochemistry Standard Curve In Biochemistry The stock solution of naoh is. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. 9.7 a focus on understanding standard curves standard curves (sometimes. Standard Curve In Biochemistry.
From microbiologynotes.org
Bacterial Growth Curve Definition, Phases and Measurement Standard Curve In Biochemistry The curve is created from the instrumental. The stock solution of naoh is. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit. Standard Curve In Biochemistry.
From www.studocu.com
biochemistry lab EXPERIMENT STANDARD MALTOSE CURVE AIM To construct Standard Curve In Biochemistry The same assay is then performed with samples of unknown concentration. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: The stock solution of naoh is. 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. A calibration curve is used to determine the. Standard Curve In Biochemistry.
From www.lifescience.net
SYBR Green qPCR with Standard Curve Protocol Standard Curve In Biochemistry A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator:. Standard Curve In Biochemistry.
From trucuulufloru.blogspot.com
Standard Curve / Standard curve for HPLC result for detection of Standard Curve In Biochemistry 0.60 g orcinol dissolved in 10 ml of 95% ethanol. 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. The curve is created from the instrumental. The stock solution of naoh is. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution. Standard Curve In Biochemistry.
From www.youtube.com
Generating Standard Curve and Determining Concentration of Unknown Standard Curve In Biochemistry The stock solution of naoh is. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. The same assay is then performed with samples of unknown. Standard Curve In Biochemistry.
From www.slideserve.com
PPT Terms Used in Biochemistry & Calculations PowerPoint Presentation Standard Curve In Biochemistry The same assay is then performed with samples of unknown concentration. The curve is created from the instrumental. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. The stock solution of naoh is. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. Alcoholic orcinol is also made in. Standard Curve In Biochemistry.
From thebiomedicalscientist.net
How to…ensure correct calibration Biomedical Scientist Standard Curve In Biochemistry 0.60 g orcinol dissolved in 10 ml of 95% ethanol. The same assay is then performed with samples of unknown concentration. 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and. Standard Curve In Biochemistry.
From www.slideserve.com
PPT CHMI 2227E Biochemistry I PowerPoint Presentation, free download Standard Curve In Biochemistry 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. The curve is created from the instrumental. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: The stock solution of naoh is. The same assay is then performed with samples of unknown concentration. 0.60. Standard Curve In Biochemistry.
From www.thepharmaeducation.com
How to Make a Calibration Curve in Excel The Pharma Education Standard Curve In Biochemistry A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. The curve is created from the instrumental. The same assay is then performed with samples of unknown concentration. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: 9.7 a. Standard Curve In Biochemistry.
From www.medicalexamprep.co.uk
Understanding the Oxygen Dissociation Curve Medical Exam Prep Standard Curve In Biochemistry The stock solution of naoh is. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: The same assay is then performed with samples of unknown concentration. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. A calibration curve is used to determine. Standard Curve In Biochemistry.
From www.chegg.com
Solved In Biochemistry, We Use A 'Bradford Assay' To Calc... Standard Curve In Biochemistry The curve is created from the instrumental. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. The stock solution of naoh is. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves). Standard Curve In Biochemistry.
From biochemistrylabreport.blogspot.com
Experiment 3 Experiment on enzyme Biochemistry Standard Curve In Biochemistry The stock solution of naoh is. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. Alcoholic orcinol is also made. Standard Curve In Biochemistry.
From www.researchgate.net
Realtime PCR standard curves of speF and adiA gene with endogenous Standard Curve In Biochemistry The stock solution of naoh is. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research. Standard Curve In Biochemistry.
From www.researchgate.net
Graph shows the standard protein estimation by biuret method Standard Curve In Biochemistry The same assay is then performed with samples of unknown concentration. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: The stock solution of naoh is. 0.60 g orcinol. Standard Curve In Biochemistry.
From www.researchgate.net
The standard curves of the realtime PCR assay. Download Scientific Standard Curve In Biochemistry 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: 0.60 g orcinol dissolved in 10 ml of 95% ethanol. The stock solution of naoh is. A calibration curve is used to determine the concentration. Standard Curve In Biochemistry.
From www.chegg.com
Solved Part 6Protein Concentrations Use the protein Standard Curve In Biochemistry 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. The stock solution of naoh is. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. The curve is created from. Standard Curve In Biochemistry.
From www.slideserve.com
PPT Terms Used in Biochemistry & Calculations PowerPoint Presentation Standard Curve In Biochemistry The stock solution of naoh is. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: 9.7 a focus on. Standard Curve In Biochemistry.
From www.reddit.com
[University biochemistry Standard curve] Hi this is my attempt at a Standard Curve In Biochemistry The same assay is then performed with samples of unknown concentration. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection,. Standard Curve In Biochemistry.
From www.slideserve.com
PPT Terms Used in Biochemistry & Calculations PowerPoint Presentation Standard Curve In Biochemistry 0.60 g orcinol dissolved in 10 ml of 95% ethanol. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. The stock solution of naoh is. The same assay is then performed with samples of unknown concentration. Alcoholic orcinol is also made in advance and stored. Standard Curve In Biochemistry.
From www.scribd.com
Glucose Standard Curve PDF Biochemistry Chemistry Standard Curve In Biochemistry A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh.. Standard Curve In Biochemistry.
From shimidoon.ir
چگونه نمودار کالیبراسیون رسم کنیم؟ * شیمی دون Standard Curve In Biochemistry Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: 0.60 g orcinol dissolved in 10 ml of 95% ethanol. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. A calibration curve is used to determine the concentration of an unknown sample, to. Standard Curve In Biochemistry.
From www.slideserve.com
PPT Introduction to Analytical Chemistry PowerPoint Presentation Standard Curve In Biochemistry The stock solution of naoh is. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. The same assay is then performed with samples of unknown concentration.. Standard Curve In Biochemistry.
From sites.chem.utoronto.ca
Statistics in Analytical Chemistry Excel™ Standard Curve In Biochemistry Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. The curve is created from the instrumental. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. 0.60 g orcinol dissolved in 10 ml of 95%. Standard Curve In Biochemistry.
From www.researchgate.net
Standard curve generated through the biochemical analysis of the Standard Curve In Biochemistry Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: The stock solution of naoh is. The curve is created from the instrumental. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. A. Standard Curve In Biochemistry.
From www.pinterest.com
Glycine Titration Curve And Its Steps Biochemistry, Curve, Medical Standard Curve In Biochemistry A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. The stock solution of naoh is. 0.60 g orcinol dissolved in 10 ml of 95% ethanol.. Standard Curve In Biochemistry.
From www.researchgate.net
Standard curves used for calculation of relative DNA concentrations of Standard Curve In Biochemistry 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. The stock solution of naoh is. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: The curve is created from the instrumental. Using a. Standard Curve In Biochemistry.
From www.youtube.com
Biochemistry/ practical lab1 / part3 construction the standard curve of Standard Curve In Biochemistry Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: The stock solution of naoh is. A calibration curve is used to determine the concentration of an unknown sample, to calculate the limit of detection, and the limit of quantitation. 0.60 g orcinol dissolved in 10 ml of 95% ethanol. The curve is created. Standard Curve In Biochemistry.
From www.researchgate.net
Absorbance spectrum of riboflavin from 320 nm to 700 nm at different pH Standard Curve In Biochemistry 9.7 a focus on understanding standard curves standard curves (sometimes called calibration curves) are incredibly important in research and. Alcoholic orcinol is also made in advance and stored in a brown bottle in the refrigerator: Using a serial dilution, describe how you would prepare 10 ml of a 1%, 0.1% and 0.01% solution of naoh. The same assay is then. Standard Curve In Biochemistry.