Is Bromine Or Chlorine A Better Leaving Group . If you take the example of the halogen. a leaving group is a weak base that can accept electrons and leave a molecule in a substitution reaction. as mentioned above, the more electronegative atoms may not be the better leaving group. a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. [the leaving group may even depart entirely to give a carbocation, an even better electrophile!] in much the same way, coordination. The best leaving groups are the. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. However, it is important to note that leaving group's ability also.
from www.masterorganicchemistry.com
If you take the example of the halogen. a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. However, it is important to note that leaving group's ability also. The best leaving groups are the. a leaving group is a weak base that can accept electrons and leave a molecule in a substitution reaction. as mentioned above, the more electronegative atoms may not be the better leaving group. [the leaving group may even depart entirely to give a carbocation, an even better electrophile!] in much the same way, coordination. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a.
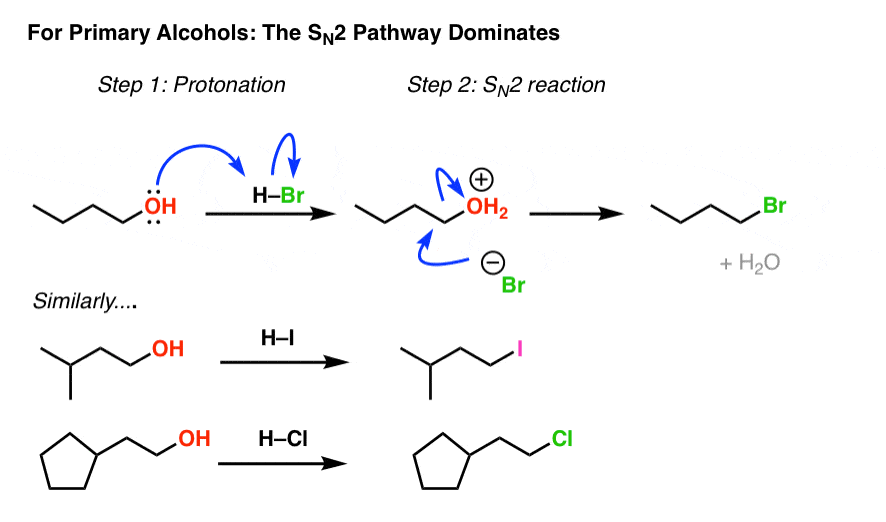
Making Alkyl Halides From Alcohols Master Organic Chemistry
Is Bromine Or Chlorine A Better Leaving Group a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. However, it is important to note that leaving group's ability also. a leaving group is a weak base that can accept electrons and leave a molecule in a substitution reaction. as mentioned above, the more electronegative atoms may not be the better leaving group. If you take the example of the halogen. [the leaving group may even depart entirely to give a carbocation, an even better electrophile!] in much the same way, coordination. The best leaving groups are the. a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Is Bromine Or Chlorine A Better Leaving Group However, it is important to note that leaving group's ability also. as mentioned above, the more electronegative atoms may not be the better leaving group. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. a leaving group is a weak base that leaves a molecule in. Is Bromine Or Chlorine A Better Leaving Group.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Is Bromine Or Chlorine A Better Leaving Group [the leaving group may even depart entirely to give a carbocation, an even better electrophile!] in much the same way, coordination. If you take the example of the halogen. a leaving group is a weak base that can accept electrons and leave a molecule in a substitution reaction. a leaving group is a weak base that leaves. Is Bromine Or Chlorine A Better Leaving Group.
From www.masterorganicchemistry.com
What makes a good leaving group? Master Organic Chemistry Is Bromine Or Chlorine A Better Leaving Group a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. If you take the example of the halogen. However, it is important to note that leaving group's ability also. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. [the leaving group may. Is Bromine Or Chlorine A Better Leaving Group.
From chem.libretexts.org
11.2 The SN2 Reaction Chemistry LibreTexts Is Bromine Or Chlorine A Better Leaving Group a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. [the leaving group may even depart entirely to give a carbocation, an even better electrophile!] in much the same way, coordination. The best. Is Bromine Or Chlorine A Better Leaving Group.
From www.spaworld.com.au
What's better for a spa Chlorine or Bromine? Is Bromine Or Chlorine A Better Leaving Group a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. [the leaving group may even depart entirely to give a carbocation, an even better electrophile!] in much the same way, coordination. as. Is Bromine Or Chlorine A Better Leaving Group.
From www.doheny.com
Bromine vs. Chlorine Everything You Need to Know Is Bromine Or Chlorine A Better Leaving Group a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. If you take the example of the halogen. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. as mentioned above, the more electronegative atoms may not be the. Is Bromine Or Chlorine A Better Leaving Group.
From byjus.com
From each of the following pairs select the compound that will react Is Bromine Or Chlorine A Better Leaving Group The best leaving groups are the. If you take the example of the halogen. as mentioned above, the more electronegative atoms may not be the better leaving group. a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. a better leaving group like bromine, and a weaker electron withdrawing group, tends. Is Bromine Or Chlorine A Better Leaving Group.
From www.clutchprep.com
SN2 Reaction Organic Chemistry Video Clutch Prep Is Bromine Or Chlorine A Better Leaving Group poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. If you take the example of the halogen. as mentioned above, the more electronegative atoms may not be the better leaving group. a leaving group is a weak base that leaves a molecule in a nucleophilic substitution. Is Bromine Or Chlorine A Better Leaving Group.
From www.masterorganicchemistry.com
What makes a good leaving group? Master Organic Chemistry Is Bromine Or Chlorine A Better Leaving Group If you take the example of the halogen. However, it is important to note that leaving group's ability also. The best leaving groups are the. a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. as mentioned above, the more electronegative atoms may not be the better leaving group. poor leaving. Is Bromine Or Chlorine A Better Leaving Group.
From www.chemistrysteps.com
SOCl2 and PBr3 Chemistry Steps Is Bromine Or Chlorine A Better Leaving Group The best leaving groups are the. as mentioned above, the more electronegative atoms may not be the better leaving group. However, it is important to note that leaving group's ability also. If you take the example of the halogen. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. . Is Bromine Or Chlorine A Better Leaving Group.
From www.chemistrysteps.com
Mesylates and Tosylates with Practice Problems Chemistry Steps Is Bromine Or Chlorine A Better Leaving Group The best leaving groups are the. However, it is important to note that leaving group's ability also. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. If you take the example of the. Is Bromine Or Chlorine A Better Leaving Group.
From www.youtube.com
Aromatic Halogenation Mechanism Chlorination, Iodination Is Bromine Or Chlorine A Better Leaving Group However, it is important to note that leaving group's ability also. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. a leaving group is a weak base that can accept electrons and leave a molecule in a substitution reaction. a leaving group is a weak base. Is Bromine Or Chlorine A Better Leaving Group.
From kpu.pressbooks.pub
7.2 SN2 Reaction Mechanisms, Energy Diagram and Stereochemistry Is Bromine Or Chlorine A Better Leaving Group a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. If you take the example of the halogen. a leaving group is a weak base that can accept electrons and leave. Is Bromine Or Chlorine A Better Leaving Group.
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromine vs. Chlorine — Master Is Bromine Or Chlorine A Better Leaving Group If you take the example of the halogen. as mentioned above, the more electronegative atoms may not be the better leaving group. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. . Is Bromine Or Chlorine A Better Leaving Group.
From chem.libretexts.org
Hydroxyl Group Substitution Chemistry LibreTexts Is Bromine Or Chlorine A Better Leaving Group [the leaving group may even depart entirely to give a carbocation, an even better electrophile!] in much the same way, coordination. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction.. Is Bromine Or Chlorine A Better Leaving Group.
From bromineinformation.weebly.com
Bromine on the Periodic Table Is Bromine Or Chlorine A Better Leaving Group However, it is important to note that leaving group's ability also. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. If you take the example of the halogen. The best leaving groups are the. [the leaving group may even depart entirely to give a carbocation, an even better electrophile!]. Is Bromine Or Chlorine A Better Leaving Group.
From blog.intheswim.com
Chlorine vs. Bromine What's the Difference? Is Bromine Or Chlorine A Better Leaving Group [the leaving group may even depart entirely to give a carbocation, an even better electrophile!] in much the same way, coordination. a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. However, it is important to note that leaving group's ability also. The best leaving groups are the. a better leaving. Is Bromine Or Chlorine A Better Leaving Group.
From blog.chloramineconsulting.com
Chlorine vs. Bromine in Indoor Pools Is Bromine Or Chlorine A Better Leaving Group The best leaving groups are the. However, it is important to note that leaving group's ability also. If you take the example of the halogen. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. a leaving group is a weak base that leaves a molecule in a nucleophilic substitution. Is Bromine Or Chlorine A Better Leaving Group.
From www.chegg.com
Solved I know Bromine is a better leaving group than Is Bromine Or Chlorine A Better Leaving Group a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. The best leaving groups are the. as mentioned above, the more electronegative atoms may not be the better leaving group. a leaving group is a weak base that can accept electrons and leave a molecule in a substitution reaction. However, it. Is Bromine Or Chlorine A Better Leaving Group.
From nhanvietluanvan.com
Group_By Two Variables A Guide To Organizing Data With R Is Bromine Or Chlorine A Better Leaving Group If you take the example of the halogen. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. [the leaving group may even depart entirely to give a carbocation,. Is Bromine Or Chlorine A Better Leaving Group.
From capoolassociation.com
Which Is Better in a Pool or Spa Chlorine or Bromine? CPA Is Bromine Or Chlorine A Better Leaving Group [the leaving group may even depart entirely to give a carbocation, an even better electrophile!] in much the same way, coordination. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. The best leaving groups are the. as mentioned above, the more electronegative atoms may not be the better. Is Bromine Or Chlorine A Better Leaving Group.
From blog.intheswim.com
Chlorine vs. Bromine What's the Difference? InTheSwim Pool Blog Is Bromine Or Chlorine A Better Leaving Group as mentioned above, the more electronegative atoms may not be the better leaving group. However, it is important to note that leaving group's ability also. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. [the leaving group may even depart entirely to give a carbocation, an. Is Bromine Or Chlorine A Better Leaving Group.
From sciencestruck.com
Bromine Vs. Chlorine Science Struck Is Bromine Or Chlorine A Better Leaving Group a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. However, it is important to note that leaving group's ability also. The best leaving groups are the. as mentioned above, the more electronegative atoms may not be the better leaving group. [the leaving group may even depart entirely to. Is Bromine Or Chlorine A Better Leaving Group.
From www.vedantu.com
Going from fluorine, chlorine, bromine to iodine the electronegativity Is Bromine Or Chlorine A Better Leaving Group a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. [the leaving group may even depart entirely to give a carbocation, an even better electrophile!] in much the same way, coordination. a leaving group is a weak base that can accept electrons and leave a molecule in a substitution. Is Bromine Or Chlorine A Better Leaving Group.
From easypoolcleaning.com
Bromine Vs Chlorine Hot Tub Sanitizer Which To Choose? Is Bromine Or Chlorine A Better Leaving Group as mentioned above, the more electronegative atoms may not be the better leaving group. If you take the example of the halogen. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. a leaving group is a weak base that can accept electrons and leave a molecule in a. Is Bromine Or Chlorine A Better Leaving Group.
From ar.inspiredpencil.com
Draw The Structure Of The Aromatic Product From The Following Reaction Is Bromine Or Chlorine A Better Leaving Group The best leaving groups are the. However, it is important to note that leaving group's ability also. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. [the leaving group may even depart entirely to give a carbocation, an even better electrophile!] in much the same way, coordination. as. Is Bromine Or Chlorine A Better Leaving Group.
From www.researchgate.net
Distribution of different group of DBPs in chlorination and bromination Is Bromine Or Chlorine A Better Leaving Group a leaving group is a weak base that can accept electrons and leave a molecule in a substitution reaction. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. The best leaving groups are the. a better leaving group like bromine, and a weaker electron withdrawing group,. Is Bromine Or Chlorine A Better Leaving Group.
From www.masterorganicchemistry.com
Leaving Group Ability Is Increased By Acid Master Organic Chemistry Is Bromine Or Chlorine A Better Leaving Group as mentioned above, the more electronegative atoms may not be the better leaving group. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. [the leaving group may even depart. Is Bromine Or Chlorine A Better Leaving Group.
From www.masterorganicchemistry.com
Making Alkyl Halides From Alcohols Master Organic Chemistry Is Bromine Or Chlorine A Better Leaving Group [the leaving group may even depart entirely to give a carbocation, an even better electrophile!] in much the same way, coordination. If you take the example of the halogen. The best leaving groups are the. However, it is important to note that leaving group's ability also. a leaving group is a weak base that can accept electrons and. Is Bromine Or Chlorine A Better Leaving Group.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Is Bromine Or Chlorine A Better Leaving Group a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. The best leaving groups are the. a leaving group is a weak base that can accept electrons and leave a molecule in a substitution reaction. If you take the example of the halogen. However, it is important to note that. Is Bromine Or Chlorine A Better Leaving Group.
From www.masterorganicchemistry.com
Making Alkyl Halides From Alcohols Master Organic Chemistry Is Bromine Or Chlorine A Better Leaving Group The best leaving groups are the. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. [the leaving group may even depart entirely to give a carbocation, an even. Is Bromine Or Chlorine A Better Leaving Group.
From www.riverpoolsandspas.com
Chlorine vs Bromine Which is Better? Is Bromine Or Chlorine A Better Leaving Group a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. as mentioned above, the more electronegative atoms may not be the better leaving group. [the leaving group may even depart entirely to. Is Bromine Or Chlorine A Better Leaving Group.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Is Bromine Or Chlorine A Better Leaving Group a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. However, it is important to note that leaving group's ability also. [the leaving group may even depart entirely to give a carbocation, an even better electrophile!] in much the same way, coordination. a leaving group is a weak base that can. Is Bromine Or Chlorine A Better Leaving Group.
From martlabpro.com
Bromine Vs Chlorine For Skin Which Is Better? MartLabPro Is Bromine Or Chlorine A Better Leaving Group as mentioned above, the more electronegative atoms may not be the better leaving group. poor leaving groups (which tend to be strong bases) can be made into better leaving groups through addition of a. a leaving group is a weak base that can accept electrons and leave a molecule in a substitution reaction. [the leaving group. Is Bromine Or Chlorine A Better Leaving Group.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes — Master Organic Chemistry Is Bromine Or Chlorine A Better Leaving Group a leaving group is a weak base that leaves a molecule in a nucleophilic substitution reaction. as mentioned above, the more electronegative atoms may not be the better leaving group. If you take the example of the halogen. a better leaving group like bromine, and a weaker electron withdrawing group, tends to favor a concerted mechanism. However,. Is Bromine Or Chlorine A Better Leaving Group.