What Is Medical Device Vigilance System . learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. 7 who will investigate an incident the manufacturer has the. this document provides clarification and detail on the medical device vigilance system under the eu directives. learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. learn how to report adverse incidents and field safety corrective actions involving medical devices to the. this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,.
from www.johner-institut.de
learn how to report adverse incidents and field safety corrective actions involving medical devices to the. this document provides clarification and detail on the medical device vigilance system under the eu directives. this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. 7 who will investigate an incident the manufacturer has the.
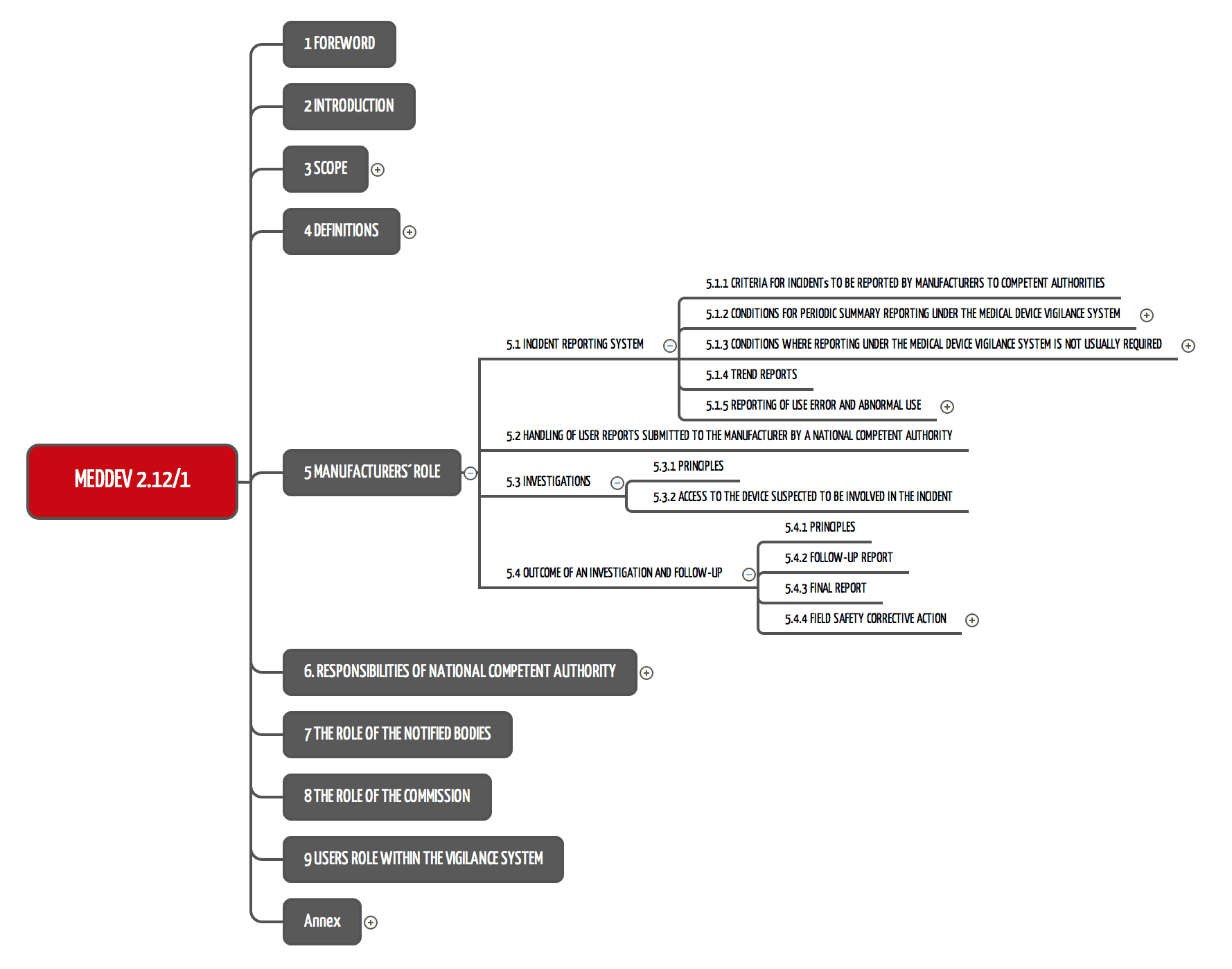
VigilanzSystem VigilanzSystem (Vigilance System) für Medizinprodukte
What Is Medical Device Vigilance System this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. learn how to report adverse incidents and field safety corrective actions involving medical devices to the. a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. this document provides clarification and detail on the medical device vigilance system under the eu directives. 7 who will investigate an incident the manufacturer has the.
From youregulate.com
EC Issues Additional Guidance on Device Vigilance System What Is Medical Device Vigilance System this document provides clarification and detail on the medical device vigilance system under the eu directives. learn how to report adverse incidents and field safety corrective actions involving medical devices to the. learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. 7 who will investigate an incident the manufacturer has the.. What Is Medical Device Vigilance System.
From www.mantrasystems.co.uk
Vigilance and PostMarket Surveillance (PMS) What Is Medical Device Vigilance System learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. 7 who will investigate an incident the manufacturer has the. learn how to report adverse incidents and field safety corrective actions involving medical devices to. What Is Medical Device Vigilance System.
From www.scribd.com
Medical Devices Vigilance System PDF Medical Device What Is Medical Device Vigilance System learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. learn how to report adverse incidents and field safety corrective actions involving medical devices to the. 7 who will investigate an incident the manufacturer has the. this document provides clarification and detail on the medical device vigilance system under the eu directives.. What Is Medical Device Vigilance System.
From www.researchgate.net
(PDF) Vigilance System Requirements Across US and EU for Medical Device What Is Medical Device Vigilance System learn how to report adverse incidents and field safety corrective actions involving medical devices to the. this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. a vigilance system is a legally. What Is Medical Device Vigilance System.
From www.quinten-md.com
PostMarket Clinical Followup (PMCF) under MDR Quinten MD What Is Medical Device Vigilance System this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. this document provides clarification and detail on. What Is Medical Device Vigilance System.
From www.slideserve.com
PPT Medical Device Vigilance PowerPoint Presentation ID783928 What Is Medical Device Vigilance System 7 who will investigate an incident the manufacturer has the. this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. a vigilance system is a legally prescribed system consisting of one or more processes through. What Is Medical Device Vigilance System.
From www.researchgate.net
Medical device vigilance system comparison between Meddev 2.121 25 vs What Is Medical Device Vigilance System a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. learn how to report adverse incidents and field safety corrective actions involving medical devices to the. 7 who will investigate an incident the manufacturer has the. this document provides clarification and detail on the medical device vigilance system under. What Is Medical Device Vigilance System.
From www.researchgate.net
(PDF) Medical device vigilance systems India, US, UK, and Australia What Is Medical Device Vigilance System a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. this document provides clarification and detail on the medical device vigilance system under the eu directives. learn how the vigilance reporting. What Is Medical Device Vigilance System.
From slideplayer.com
Vigilance of medical devices and IVDs in the South East Asia Region What Is Medical Device Vigilance System this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. this document provides clarification and detail on the medical device vigilance system under the eu directives. learn how to report adverse incidents and field. What Is Medical Device Vigilance System.
From dokumen.tips
(PDF) The Egyptian Guideline for Medical Device Vigilance System What Is Medical Device Vigilance System this document provides clarification and detail on the medical device vigilance system under the eu directives. learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. 7 who will investigate an incident the manufacturer has the. learn how to report adverse incidents and field safety corrective actions involving medical devices. What Is Medical Device Vigilance System.
From www.aplyon.com
Vigilance Procedure What Is Medical Device Vigilance System a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. this document provides clarification and detail on the medical device vigilance system under the eu directives. this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. learn how vigilance and post. What Is Medical Device Vigilance System.
From www.johner-institut.de
VigilanzSystem VigilanzSystem (Vigilance System) für Medizinprodukte What Is Medical Device Vigilance System this document provides clarification and detail on the medical device vigilance system under the eu directives. learn how to report adverse incidents and field safety corrective actions involving medical devices to the. a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. learn how vigilance and post market. What Is Medical Device Vigilance System.
From www.i3cglobal.com
Medical Device Vigilance System Consultants What Is Medical Device Vigilance System learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. a vigilance system is a legally prescribed. What Is Medical Device Vigilance System.
From www.scribd.com
The Egyptian Guideline For Medical Device Vigilance System 2013 With What Is Medical Device Vigilance System this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. learn how vigilance and post market. What Is Medical Device Vigilance System.
From www.productlifegroup.com
Medical Devices Guidance for Manufacturers on Vigilance What Is Medical Device Vigilance System learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. learn how to report adverse incidents and field safety corrective actions involving medical devices to the. 7 who will investigate an incident the manufacturer has the. a vigilance system is a legally prescribed system consisting of one or more processes through which. What Is Medical Device Vigilance System.
From www.scribd.com
Guidance On The Vigilance System For CEmarked Medical Devices PDF What Is Medical Device Vigilance System learn how to report adverse incidents and field safety corrective actions involving medical devices to the. learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. a vigilance system is a legally prescribed system. What Is Medical Device Vigilance System.
From kvalito.ch
Vigilance from a Medical Device Perspective Kvalito What Is Medical Device Vigilance System 7 who will investigate an incident the manufacturer has the. a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. this document provides clarification and detail on the medical device vigilance system under the. What Is Medical Device Vigilance System.
From www.asphalion.com
How to setup your PostMarket Surveillance and Vigilance system for What Is Medical Device Vigilance System learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. learn how to report adverse incidents and field safety corrective actions involving medical devices to the. this document provides clarification and detail on the medical device vigilance system under the eu directives. learn how the vigilance reporting requirements for. What Is Medical Device Vigilance System.
From www.scribd.com
Rules on Vigilance of Medical Devices.pdf Medical Device Adverse Effect What Is Medical Device Vigilance System learn how to report adverse incidents and field safety corrective actions involving medical devices to the. a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. 7 who will investigate an incident. What Is Medical Device Vigilance System.
From gimd.de
Medical Device Vigilance GIMD GmbH What Is Medical Device Vigilance System a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. 7 who will investigate an incident the manufacturer has the. learn how to report adverse incidents and field safety corrective actions involving medical devices to the. learn how vigilance and post market surveillance (pms) are distinguished in the eu. What Is Medical Device Vigilance System.
From www.slideserve.com
PPT Medical Device Vigilance PowerPoint Presentation, free download What Is Medical Device Vigilance System a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. 7 who will investigate an incident the. What Is Medical Device Vigilance System.
From omcmedical.com
Vigilance Terms & Concepts (EU) 2017/745 on Medical Devices What Is Medical Device Vigilance System a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. this document provides clarification and detail on the medical device vigilance system under the eu directives. learn how the vigilance reporting. What Is Medical Device Vigilance System.
From kvalito.ch
Vigilance from a Medical Device Perspective Kvalito What Is Medical Device Vigilance System this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. learn how to report adverse incidents and field safety corrective actions involving medical devices to the. a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. learn how vigilance and post. What Is Medical Device Vigilance System.
From quality.eleapsoftware.com
Shielding Patient Safety in the Medical Device Sector MEDDEV Vigilance What Is Medical Device Vigilance System 7 who will investigate an incident the manufacturer has the. a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. this document clarifies important terms and concepts for the vigilance requirements under the eu. What Is Medical Device Vigilance System.
From www.researchgate.net
(PDF) Medical device vigilance systems India, US, UK, and Australia What Is Medical Device Vigilance System this document provides clarification and detail on the medical device vigilance system under the eu directives. this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. learn how the vigilance reporting. What Is Medical Device Vigilance System.
From www.scribd.com
Guidelines on Medical Devices Vigilance System PDF What Is Medical Device Vigilance System a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. this document provides clarification and detail on the medical device vigilance system under the eu directives. learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. learn how the vigilance reporting. What Is Medical Device Vigilance System.
From omcmedical.com
Vigilance Terms & Concepts (EU) 2017/745 on Medical Devices What Is Medical Device Vigilance System learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. learn how to report adverse incidents and field safety corrective actions involving medical devices to the. 7 who will investigate an incident the manufacturer has the. a vigilance system is a legally prescribed system consisting of one or more processes through which. What Is Medical Device Vigilance System.
From mavenprofserv.com
Vigilance System FSCA and Medical Devices Reporting Solutions What Is Medical Device Vigilance System learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. learn how to report adverse incidents and field safety corrective actions involving medical devices to the. 7 who will investigate an incident the manufacturer has the. this document clarifies important terms and concepts for the vigilance requirements under the eu. What Is Medical Device Vigilance System.
From www.slideserve.com
PPT Latin America APEC Funded Medical Device Regulatory Seminar What Is Medical Device Vigilance System a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. this document provides clarification and detail on the medical device vigilance system under the eu directives. learn how to report adverse. What Is Medical Device Vigilance System.
From www.slideserve.com
PPT Vigilance guidance for specific devices Tony Sant/Andy Crosbie What Is Medical Device Vigilance System this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. this document provides clarification and detail on the medical device vigilance system under the eu directives. a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. 7 who will investigate an incident. What Is Medical Device Vigilance System.
From omcmedical.com
Vigilance Reporting in EU MDR OMC Medical What Is Medical Device Vigilance System a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. learn how vigilance and post market surveillance (pms) are distinguished in the eu medical device regulation (eu. 7 who will investigate an incident the manufacturer has the. this document clarifies important terms and concepts for the vigilance requirements under. What Is Medical Device Vigilance System.
From www.ebeling-assoc.com
Medical Device Vigilance Dr. Ebeling & Assoc. GmbH What Is Medical Device Vigilance System this document clarifies important terms and concepts for the vigilance requirements under the eu regulation on medical. learn how the vigilance reporting requirements for medical devices differ across mdsap participating countries and. 7 who will investigate an incident the manufacturer has the. this document provides clarification and detail on the medical device vigilance system under the eu. What Is Medical Device Vigilance System.
From www.academia.edu
(PDF) EDI system definition for a European medical device vigilance What Is Medical Device Vigilance System 7 who will investigate an incident the manufacturer has the. this document provides clarification and detail on the medical device vigilance system under the eu directives. a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. learn how vigilance and post market surveillance (pms) are distinguished in the eu. What Is Medical Device Vigilance System.
From www.eke-electronics.com
Vigilance Control System EKEElectronics What Is Medical Device Vigilance System a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. this document provides clarification and detail on the medical device vigilance system under the eu directives. 7 who will investigate an incident the manufacturer has the. learn how to report adverse incidents and field safety corrective actions involving medical. What Is Medical Device Vigilance System.
From www.pharmafilepv.ie
Medical Device Vigilance Pharmafile PV Services Limited What Is Medical Device Vigilance System a vigilance system is a legally prescribed system consisting of one or more processes through which manufacturers (e.g.,. this document provides clarification and detail on the medical device vigilance system under the eu directives. learn how to report adverse incidents and field safety corrective actions involving medical devices to the. 7 who will investigate an incident the. What Is Medical Device Vigilance System.