Does Water Vapor Increase With Altitude . The greenhouse effect of water vapor is enhanced. The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? It seems like one of those basic science facts: If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. Estimations of the amount of. When you go to a higher altitude, the boiling point is lower not because the. Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. The boiling point of a liquid is the temperature such that the liquid's vapor pressure is equal to the atmospheric pressure. Water vapor is the dominant greenhouse gas in our atmosphere. It depends on where you’re doing the boiling.
from www.e-education.psu.edu
The boiling point of a liquid is the temperature such that the liquid's vapor pressure is equal to the atmospheric pressure. It depends on where you’re doing the boiling. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. Estimations of the amount of. Water vapor is the dominant greenhouse gas in our atmosphere. It seems like one of those basic science facts: The greenhouse effect of water vapor is enhanced. When you go to a higher altitude, the boiling point is lower not because the.
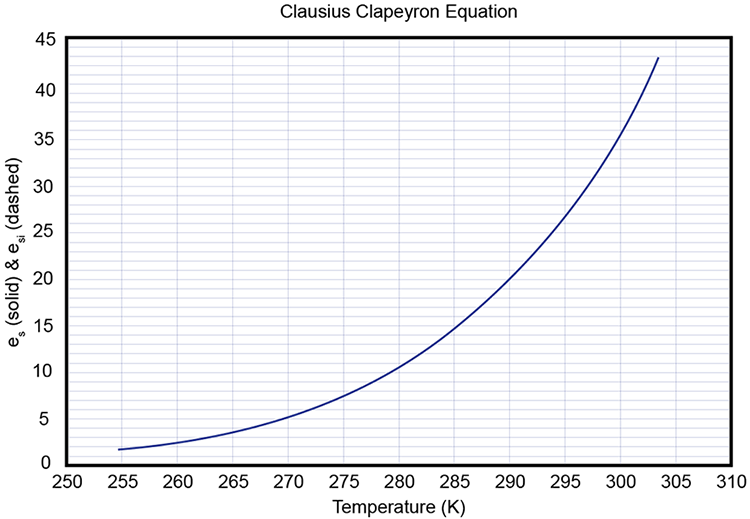
3.3 Phase Diagram for Water Vapor Clausius Clapeyron Equation METEO
Does Water Vapor Increase With Altitude Water boils at 212 degrees fahrenheit (100 degrees celsius), right? In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. It depends on where you’re doing the boiling. It seems like one of those basic science facts: The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. The greenhouse effect of water vapor is enhanced. When you go to a higher altitude, the boiling point is lower not because the. Water vapor is the dominant greenhouse gas in our atmosphere. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. The boiling point of a liquid is the temperature such that the liquid's vapor pressure is equal to the atmospheric pressure. Estimations of the amount of.
From climate.nasa.gov
Steamy Relationships How Atmospheric Water Vapor Amplifies Earth's Does Water Vapor Increase With Altitude It depends on where you’re doing the boiling. The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. When you go to a higher altitude, the boiling point is lower not because the. Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. Water boils at 212. Does Water Vapor Increase With Altitude.
From www.researchgate.net
Air pressure, water vapour concentration and temperature variation at Does Water Vapor Increase With Altitude If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. Estimations of the amount of. Water boils at 212 degrees fahrenheit (100. Does Water Vapor Increase With Altitude.
From airs.jpl.nasa.gov
AIRS Today's water vapor, 500700 millibar Does Water Vapor Increase With Altitude If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. Estimations of the amount of. It seems like one of those basic science facts: The boiling point of a liquid is the temperature such that the liquid's vapor pressure is equal to the. Does Water Vapor Increase With Altitude.
From scienceblogs.com
Water in Space What Happens? ScienceBlogs Does Water Vapor Increase With Altitude Water boils at 212 degrees fahrenheit (100 degrees celsius), right? The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. The boiling point of a liquid is the temperature such that the liquid's vapor pressure is equal to the atmospheric pressure. Water vapor is the dominant greenhouse gas in our atmosphere.. Does Water Vapor Increase With Altitude.
From www.researchgate.net
Latitudealtitude sections of the (a) water vapor content (units Does Water Vapor Increase With Altitude If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. It seems like one of those basic science facts: It depends on where you’re doing the boiling. Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. The greenhouse. Does Water Vapor Increase With Altitude.
From www.slideserve.com
PPT Moisture Relationships PowerPoint Presentation, free download Does Water Vapor Increase With Altitude When you go to a higher altitude, the boiling point is lower not because the. The greenhouse effect of water vapor is enhanced. Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions. Does Water Vapor Increase With Altitude.
From www.researchgate.net
Same as Fig. 7 but for water vapor profiles between the altitudes 1320 Does Water Vapor Increase With Altitude Estimations of the amount of. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? Water vapor is the dominant greenhouse gas in our atmosphere. The concentration of water vapour decreases with increasing altitude up to over 20 km,. Does Water Vapor Increase With Altitude.
From sites.gsu.edu
Lab 3 The Troposphere Does Water Vapor Increase With Altitude The greenhouse effect of water vapor is enhanced. It seems like one of those basic science facts: Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. Estimations of the amount of. It depends on where you’re doing the boiling. Water vapor is the dominant greenhouse gas in our atmosphere. Water boils at 212 degrees fahrenheit. Does Water Vapor Increase With Altitude.
From www.science.org
Water Vapor in the Lower Stratosphere Science Does Water Vapor Increase With Altitude The boiling point of a liquid is the temperature such that the liquid's vapor pressure is equal to the atmospheric pressure. It seems like one of those basic science facts: When you go to a higher altitude, the boiling point is lower not because the. If the air contains water vapor, then cooling of the air can cause the water. Does Water Vapor Increase With Altitude.
From climate.nasa.gov
Steamy Relationships How Atmospheric Water Vapor Supercharges Earth's Does Water Vapor Increase With Altitude If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. It depends on where you’re doing the boiling. Water vapor was primarily distributed below. Does Water Vapor Increase With Altitude.
From eos.org
Uncovering the Hidden Secrets of Water Vapor Eos Does Water Vapor Increase With Altitude The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. Estimations of the amount of. The greenhouse effect of water vapor is enhanced. It depends on where you’re doing the. Does Water Vapor Increase With Altitude.
From www.e-education.psu.edu
3.3 Phase Diagram for Water Vapor Clausius Clapeyron Equation METEO Does Water Vapor Increase With Altitude Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. Estimations of the amount of. Water vapor is the dominant greenhouse gas in our atmosphere. It seems like one of those basic science facts: When you go to a higher altitude, the boiling point is lower not because the. It depends on where you’re doing the. Does Water Vapor Increase With Altitude.
From www.researchgate.net
1 The atmospheric density and pressure distribution. Download Does Water Vapor Increase With Altitude The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. It seems like one of those basic science facts: The greenhouse effect of water vapor is enhanced. It depends on where you’re doing the boiling. The boiling point of a liquid is the temperature such that the liquid's vapor pressure is. Does Water Vapor Increase With Altitude.
From www.diabetesinc.net
phase diagram of water Diabetes Inc. Does Water Vapor Increase With Altitude Water boils at 212 degrees fahrenheit (100 degrees celsius), right? When you go to a higher altitude, the boiling point is lower not because the. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. The concentration of water vapour decreases with increasing altitude up to over 20 km,. Does Water Vapor Increase With Altitude.
From www.weforum.org
How rising water vapour in the atmosphere fuels climate change World Does Water Vapor Increase With Altitude In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. Water vapor was primarily distributed below 2.0 km and generally decreases with. Does Water Vapor Increase With Altitude.
From geomodderfied.weebly.com
Relationship between Temperature, Pressure, Density and Humidity Does Water Vapor Increase With Altitude Estimations of the amount of. The boiling point of a liquid is the temperature such that the liquid's vapor pressure is equal to the atmospheric pressure. If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. In fact, water will boil at about. Does Water Vapor Increase With Altitude.
From www.researchgate.net
Water vapour pressure (hPa) Vs. HAPS altitude (km) Download Does Water Vapor Increase With Altitude Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas.. Does Water Vapor Increase With Altitude.
From www.researchgate.net
Timeheight cross section of meridional water vapor flux under (left Does Water Vapor Increase With Altitude Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. When you go to a higher altitude, the boiling point is lower not because the. Water vapor is the dominant greenhouse gas in our atmosphere. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? It seems like one of those basic science facts: The boiling. Does Water Vapor Increase With Altitude.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Does Water Vapor Increase With Altitude If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? It seems like one of those basic science facts: Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude.. Does Water Vapor Increase With Altitude.
From www.researchgate.net
Average water vapor densities and their std at different altitudes for Does Water Vapor Increase With Altitude Water vapor is the dominant greenhouse gas in our atmosphere. Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. When you go to a higher altitude, the boiling point is lower not because the. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? The concentration of water vapour decreases with increasing altitude up to. Does Water Vapor Increase With Altitude.
From www.dreamstime.com
Boiling Point of Water in Different Altitude Meter Levels Outline Does Water Vapor Increase With Altitude In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? The greenhouse effect of water vapor is enhanced. If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no. Does Water Vapor Increase With Altitude.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Does Water Vapor Increase With Altitude The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude.. Does Water Vapor Increase With Altitude.
From www.researchgate.net
(a) Variation of water vapor density (WVD) at 15 altitudes over Hong Does Water Vapor Increase With Altitude Water vapor is the dominant greenhouse gas in our atmosphere. The boiling point of a liquid is the temperature such that the liquid's vapor pressure is equal to the atmospheric pressure. When you go to a higher altitude, the boiling point is lower not because the. Estimations of the amount of. Water boils at 212 degrees fahrenheit (100 degrees celsius),. Does Water Vapor Increase With Altitude.
From scied.ucar.edu
Change in the Atmosphere with Altitude Center for Science Education Does Water Vapor Increase With Altitude The greenhouse effect of water vapor is enhanced. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. When. Does Water Vapor Increase With Altitude.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel Does Water Vapor Increase With Altitude It seems like one of those basic science facts: Water vapor is the dominant greenhouse gas in our atmosphere. The boiling point of a liquid is the temperature such that the liquid's vapor pressure is equal to the atmospheric pressure. The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. The. Does Water Vapor Increase With Altitude.
From www.researchgate.net
(a) Variation of water vapor density (WVD) at 15 altitudes over Hong Does Water Vapor Increase With Altitude It depends on where you’re doing the boiling. The greenhouse effect of water vapor is enhanced. Water vapor is the dominant greenhouse gas in our atmosphere. If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. It seems like one of those basic. Does Water Vapor Increase With Altitude.
From www.slideserve.com
PPT Chapter 18 Water, Clouds, and Precipitation PowerPoint Does Water Vapor Increase With Altitude Water vapor is the dominant greenhouse gas in our atmosphere. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. It seems like one of those basic science facts: Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. Water boils at 212 degrees fahrenheit. Does Water Vapor Increase With Altitude.
From www.atmo.arizona.edu
Supplementary reading the troposphere & stratosphere Does Water Vapor Increase With Altitude Estimations of the amount of. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. It depends on where you’re doing the boiling. The boiling point of a liquid is the temperature such that the liquid's vapor pressure is. Does Water Vapor Increase With Altitude.
From socratic.org
How does atmospheric pressure change with altitude? Socratic Does Water Vapor Increase With Altitude The greenhouse effect of water vapor is enhanced. When you go to a higher altitude, the boiling point is lower not because the. Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. It seems like one of those basic science facts: It depends on where you’re doing the boiling. Water boils at 212 degrees fahrenheit. Does Water Vapor Increase With Altitude.
From courses.lumenlearning.com
The Hydrologic Cycle Biology for Majors II Does Water Vapor Increase With Altitude If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. The boiling point of a liquid is the temperature such that the liquid's vapor pressure is equal to the atmospheric pressure. In fact, water will boil at about 202 degrees in denver, due. Does Water Vapor Increase With Altitude.
From agupubs.onlinelibrary.wiley.com
Water Vapor, Clouds, and Saturation in the Tropical Tropopause Layer Does Water Vapor Increase With Altitude Water boils at 212 degrees fahrenheit (100 degrees celsius), right? The greenhouse effect of water vapor is enhanced. The boiling point of a liquid is the temperature such that the liquid's vapor pressure is equal to the atmospheric pressure. Estimations of the amount of. When you go to a higher altitude, the boiling point is lower not because the. In. Does Water Vapor Increase With Altitude.
From sealevel.jpl.nasa.gov
Air & Water Understanding Climate Ocean Surface Topography from Space Does Water Vapor Increase With Altitude The greenhouse effect of water vapor is enhanced. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. It depends on where you’re doing the boiling. The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. If the air contains. Does Water Vapor Increase With Altitude.
From andymaypetrophysicist.com
Does Global Warming increase total atmospheric water vapor (TPW Does Water Vapor Increase With Altitude If the air contains water vapor, then cooling of the air can cause the water to condense, and the air no longer functions as an ideal gas. The greenhouse effect of water vapor is enhanced. The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. Water vapor was primarily distributed below. Does Water Vapor Increase With Altitude.
From www.researchgate.net
Change of water vapor in Domain A during the 5hour evaluation period Does Water Vapor Increase With Altitude Estimations of the amount of. Water vapor was primarily distributed below 2.0 km and generally decreases with increasing altitude. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. Water. Does Water Vapor Increase With Altitude.
From www.britannica.com
How water vapour enters Earth's atmosphere explained Britannica Does Water Vapor Increase With Altitude When you go to a higher altitude, the boiling point is lower not because the. In fact, water will boil at about 202 degrees in denver, due to the lower air pressure at such high elevations. The concentration of water vapour decreases with increasing altitude up to over 20 km, where some increase may occur. The greenhouse effect of water. Does Water Vapor Increase With Altitude.