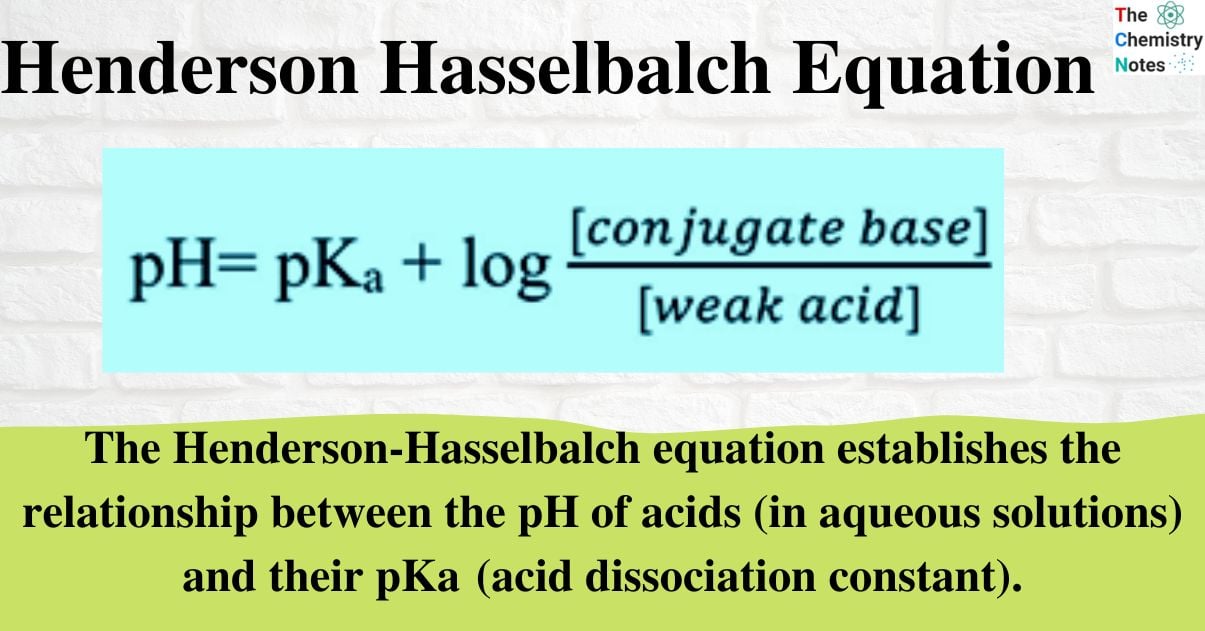
For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is . Poh = 3.99 + log10 = 4.32. Poh = pkb + log10. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of.
from scienceinfo.com
Poh = pkb + log10. Poh = 3.99 + log10 = 4.32. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base.
Henderson Hasselbalch Equation Estimating The pH of Buffers
For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = pkb + log10. Poh = 3.99 + log10 = 4.32. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = pkb + log10. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base.
From www.studypool.com
SOLUTION Henderson hasselbalch equation Studypool For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = 3.99 + log10 = 4.32. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = pkb + log10. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.slideserve.com
PPT The HendersonHasselbalch Equation PowerPoint Presentation, free For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = pkb + log10. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = 3.99 + log10 = 4.32. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.slideserve.com
PPT REGULATION OF ACIDBASE & ELECTROLYTES PowerPoint Presentation For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = pkb + log10. Poh = 3.99 + log10 = 4.32. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From sciencenotes.org
Henderson Hasselbalch Equation and Examples For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = 3.99 + log10 = 4.32. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = pkb + log10. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.numerade.com
SOLVED Use the HendersonHasselbalch equation to perform the following For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = 3.99 + log10 = 4.32. Poh = pkb + log10. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.numerade.com
SOLVED Which expression is the correct form of the Henderson For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = pkb + log10. Poh = 3.99 + log10 = 4.32. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID676897 For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = pkb + log10. Poh = 3.99 + log10 = 4.32. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.numerade.com
SOLVED How do you use the HendersonHasselbalch equation to calculate For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = 3.99 + log10 = 4.32. Poh = pkb + log10. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.slideserve.com
PPT The HendersonHasselbalch Equation PowerPoint Presentation, free For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = pkb + log10. Poh = 3.99 + log10 = 4.32. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.youtube.com
HendersonHasselbalch Equation Acids & Bases Chemistry YouTube For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = 3.99 + log10 = 4.32. Poh = pkb + log10. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From misteranthony.weebly.com
Applications of Acids & Bases For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = pkb + log10. Poh = 3.99 + log10 = 4.32. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.numerade.com
SOLVED Using the HendersonHasselbalch equation, demonstrate that the For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = 3.99 + log10 = 4.32. Poh = pkb + log10. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From microbenotes.com
Henderson Hasselbalch Equation Microbe Notes For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = pkb + log10. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = 3.99 + log10 = 4.32. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.studypool.com
SOLUTION Henderson hasselbalch equation Studypool For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = 3.99 + log10 = 4.32. Poh = pkb + log10. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.youtube.com
The Henderson Hasselbach Equation and Amino Acid pH Evolution YouTube For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = 3.99 + log10 = 4.32. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = pkb + log10. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.youtube.com
pH and pKa relationship HendersonHasselbalch equation YouTube For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = 3.99 + log10 = 4.32. Poh = pkb + log10. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.youtube.com
Henderson Hasselbalch Equation YouTube For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = 3.99 + log10 = 4.32. Poh = pkb + log10. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From scienceinfo.com
Henderson Hasselbalch Equation Estimating The pH of Buffers For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = 3.99 + log10 = 4.32. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = pkb + log10. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.slideshare.net
Henderson Hasselbalch Equation For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = pkb + log10. Poh = 3.99 + log10 = 4.32. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.slideshare.net
Henderson Hasselbalch Equation For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = pkb + log10. Poh = 3.99 + log10 = 4.32. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From thomasabsect.blogspot.com
Henderson Hasselbalch Equation For Weak Acids PPT The Henderson For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = 3.99 + log10 = 4.32. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = pkb + log10. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.slideshare.net
Henderson Hasselbalch Equation For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = pkb + log10. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = 3.99 + log10 = 4.32. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.youtube.com
The HendersonHasselbalch Equation YouTube For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = 3.99 + log10 = 4.32. Poh = pkb + log10. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.chemistrylearner.com
HendersonHasselbalch Equation Derivation and Problems For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = 3.99 + log10 = 4.32. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = pkb + log10. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.youtube.com
Henderson Hasselbalch Equation YouTube For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = 3.99 + log10 = 4.32. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = pkb + log10. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.slideshare.net
Henderson Hasselbalch Equation For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = 3.99 + log10 = 4.32. Poh = pkb + log10. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.slideserve.com
PPT The HendersonHasselbalch Equation PowerPoint Presentation ID For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = 3.99 + log10 = 4.32. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = pkb + log10. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From microbenotes.com
Henderson Hasselbalch Equation Microbe Notes For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = 3.99 + log10 = 4.32. Poh = pkb + log10. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.slideserve.com
PPT The HendersonHasselbalch Equation PowerPoint Presentation ID For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = pkb + log10. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = 3.99 + log10 = 4.32. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.youtube.com
Intro to Buffer Solutions Henderson Hasselbalch Equation and Acid Base For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = 3.99 + log10 = 4.32. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = pkb + log10. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From rk.md
HendersonHasselbalch Equation RK.MD For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = 3.99 + log10 = 4.32. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = pkb + log10. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From stephanie2867.blogspot.com
Henderson Hasselbalch Equation Examples Stephanie For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = 3.99 + log10 = 4.32. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = pkb + log10. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.sliderbase.com
Buffers Presentation Chemistry For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = 3.99 + log10 = 4.32. Poh = pkb + log10. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.numerade.com
SOLVED 1 Starting from the reaction for weak base (see below) the For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = pkb + log10. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. Poh = 3.99 + log10 = 4.32. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.
From www.numerade.com
Which of the following mathematical expressions is the Henderson For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is Poh = 3.99 + log10 = 4.32. They are typically weak acids or bases whose changes in color correspond to deprotonation or protonation of. Poh = pkb + log10. The henderson hasselbalch equation finds the ph of a weak acid or poh of a weak base. For Acid-Base Indicator In The Correct Form Of Henderson-Hasselbalch Equation Is.