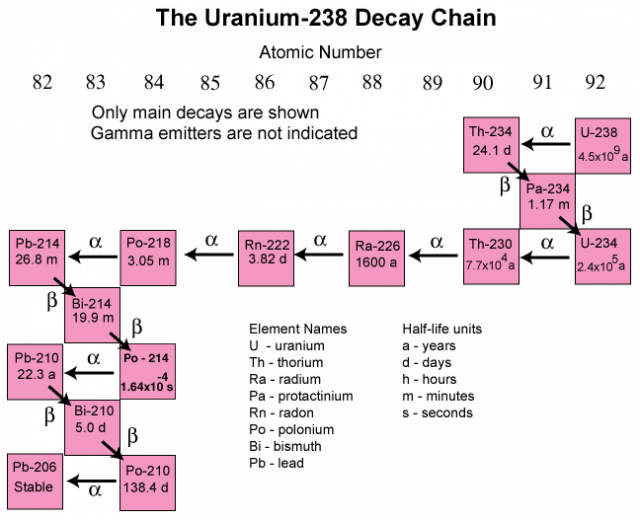
How Does Radium Decay . That morphing process is called radioactive decay. Like radium, many of these decay products also release radiation and form other. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. One of the products of radium decay is radon, the heaviest noble gas; As radium decays, it releases radiation and forms decay products. This decay process is the chief source of that element. This quickly increases to 160 w/kg within a few days through the establishment of a secular. Radium decays to produce radon gas. And decay reactions almost always involve giving off energy, radiation and more tiny particles. In radioactive decay, there are lots of ways an unstable atom’s nucleus can transform to make it more stable — and smaller. Radium is not a stable element. To reach a lower, more stable energy level, it releases energy in the form of radiation. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. The radioactive decay of pure radium releases an energy output of 28 w/kg;
from www.epa.gov
Radium decays to produce radon gas. To reach a lower, more stable energy level, it releases energy in the form of radiation. The radioactive decay of pure radium releases an energy output of 28 w/kg; Radium is not a stable element. That morphing process is called radioactive decay. This decay process is the chief source of that element. In radioactive decay, there are lots of ways an unstable atom’s nucleus can transform to make it more stable — and smaller. Like radium, many of these decay products also release radiation and form other. In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable.
Radioactive Decay US EPA
How Does Radium Decay Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. Like radium, many of these decay products also release radiation and form other. In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. Radium decays to produce radon gas. Radium is not a stable element. To reach a lower, more stable energy level, it releases energy in the form of radiation. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. As radium decays, it releases radiation and forms decay products. This quickly increases to 160 w/kg within a few days through the establishment of a secular. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. That morphing process is called radioactive decay. And decay reactions almost always involve giving off energy, radiation and more tiny particles. This decay process is the chief source of that element. One of the products of radium decay is radon, the heaviest noble gas; In radioactive decay, there are lots of ways an unstable atom’s nucleus can transform to make it more stable — and smaller. The radioactive decay of pure radium releases an energy output of 28 w/kg;
From www.slideserve.com
PPT Nuclear Chemistry PowerPoint Presentation, free download ID2426754 How Does Radium Decay The radioactive decay of pure radium releases an energy output of 28 w/kg; This decay process is the chief source of that element. To reach a lower, more stable energy level, it releases energy in the form of radiation. And decay reactions almost always involve giving off energy, radiation and more tiny particles. That morphing process is called radioactive decay.. How Does Radium Decay.
From www.researchgate.net
Decay scheme for Radium223. Download Scientific Diagram How Does Radium Decay That morphing process is called radioactive decay. In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. And decay reactions almost always involve giving off energy, radiation and more tiny particles. To reach a lower, more stable energy level, it releases energy in the form of radiation. Radioactivity occurs when an atom. How Does Radium Decay.
From www.sciencephoto.com
Nuclear decay chain, radium series Stock Image C015/2759 Science How Does Radium Decay To reach a lower, more stable energy level, it releases energy in the form of radiation. That morphing process is called radioactive decay. In radioactive decay, there are lots of ways an unstable atom’s nucleus can transform to make it more stable — and smaller. And decay reactions almost always involve giving off energy, radiation and more tiny particles. This. How Does Radium Decay.
From stock.adobe.com
radium226 nucleus undergoes alpha decay to form radon222 Stock Vector How Does Radium Decay The radioactive decay of pure radium releases an energy output of 28 w/kg; And decay reactions almost always involve giving off energy, radiation and more tiny particles. To reach a lower, more stable energy level, it releases energy in the form of radiation. As radium decays, it releases radiation and forms decay products. Like radium, many of these decay products. How Does Radium Decay.
From proper-cooking.info
Radioactive Decay Chart How Does Radium Decay In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. That morphing process is called radioactive decay. As radium decays, it releases radiation and forms decay products. This decay process is the chief source of that element. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its. How Does Radium Decay.
From www.euronuclear.org
Radioactivity ENS How Does Radium Decay Radium is not a stable element. To reach a lower, more stable energy level, it releases energy in the form of radiation. This quickly increases to 160 w/kg within a few days through the establishment of a secular. As radium decays, it releases radiation and forms decay products. Radioactivity occurs when an atom has an excess of energy, mass, or. How Does Radium Decay.
From www.slideserve.com
PPT Radioactive Decay of Plutonium239 Over the course of 80.8 How Does Radium Decay As radium decays, it releases radiation and forms decay products. Radium is not a stable element. One of the products of radium decay is radon, the heaviest noble gas; Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. And decay reactions almost always involve giving off energy, radiation and more tiny particles.. How Does Radium Decay.
From www.slideshare.net
Nuclear Decay How Does Radium Decay In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. To reach a lower, more stable energy level, it releases energy in the form of radiation. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. Like radium, many of these decay products also release. How Does Radium Decay.
From socratic.org
Radiation emitted during radon's decay process was discovered to be How Does Radium Decay In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. To reach a lower, more stable energy level, it releases energy in the form of radiation. And decay reactions almost always involve giving off energy, radiation and more tiny particles. Radium decays to produce radon gas. This quickly increases to 160 w/kg. How Does Radium Decay.
From sciencedrill.com
Calculating and Alpha Decay Energy or QValue Science Drill How Does Radium Decay The radioactive decay of pure radium releases an energy output of 28 w/kg; This decay process is the chief source of that element. Like radium, many of these decay products also release radiation and form other. Radium is not a stable element. In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals.. How Does Radium Decay.
From data.allenai.org
radioactive decay as a measure of age (lesson 0283) TQA explorer How Does Radium Decay Radium is not a stable element. Radium decays to produce radon gas. Like radium, many of these decay products also release radiation and form other. In radioactive decay, there are lots of ways an unstable atom’s nucleus can transform to make it more stable — and smaller. To reach a lower, more stable energy level, it releases energy in the. How Does Radium Decay.
From socratic.org
Question a7d58 Socratic How Does Radium Decay Like radium, many of these decay products also release radiation and form other. That morphing process is called radioactive decay. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. In radioactive decay, there are lots. How Does Radium Decay.
From slideplayer.com
Lesson 1 Nuclear Radiation. ppt download How Does Radium Decay Like radium, many of these decay products also release radiation and form other. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. And decay reactions almost always involve giving off energy, radiation and more tiny particles. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the. How Does Radium Decay.
From www.coursehero.com
HalfLife and Activity Physics Course Hero How Does Radium Decay To reach a lower, more stable energy level, it releases energy in the form of radiation. This quickly increases to 160 w/kg within a few days through the establishment of a secular. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. The radioactive decay of pure radium releases an energy output of. How Does Radium Decay.
From www.naturphilosophie.co.uk
Radioactivity and the Background of Dancing Particles NaturPhilosophie How Does Radium Decay The radioactive decay of pure radium releases an energy output of 28 w/kg; Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. Radium decays to produce radon gas. Radium is not a stable element. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. That. How Does Radium Decay.
From www.toppr.com
Radioactivity Law of Radioactive Decay, Decay Rate, HalfMean Life, Q&A How Does Radium Decay As radium decays, it releases radiation and forms decay products. In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. And decay reactions almost always involve giving off energy, radiation and more tiny particles. In radioactive decay, there are lots of ways an unstable atom’s nucleus can transform to make it more. How Does Radium Decay.
From theconversation.com
Radium revealed 120 years since Curies found the most radioactive How Does Radium Decay In radioactive decay, there are lots of ways an unstable atom’s nucleus can transform to make it more stable — and smaller. As radium decays, it releases radiation and forms decay products. Like radium, many of these decay products also release radiation and form other. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its. How Does Radium Decay.
From www.youtube.com
Radium Decay YouTube How Does Radium Decay As radium decays, it releases radiation and forms decay products. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. This quickly increases to 160 w/kg within a few days through the establishment of a secular.. How Does Radium Decay.
From www.researchgate.net
Decay scheme for Radium223. Download Scientific Diagram How Does Radium Decay This quickly increases to 160 w/kg within a few days through the establishment of a secular. To reach a lower, more stable energy level, it releases energy in the form of radiation. Like radium, many of these decay products also release radiation and form other. The radioactive decay of pure radium releases an energy output of 28 w/kg; One of. How Does Radium Decay.
From physicsopenlab.org
Radon PhysicsOpenLab How Does Radium Decay Like radium, many of these decay products also release radiation and form other. This decay process is the chief source of that element. The radioactive decay of pure radium releases an energy output of 28 w/kg; And decay reactions almost always involve giving off energy, radiation and more tiny particles. Radium decays to produce radon gas. As radium decays, it. How Does Radium Decay.
From passeportmtletudiant.com
Explainer Radiation and radioactive decay Passeport Montreal How Does Radium Decay One of the products of radium decay is radon, the heaviest noble gas; This decay process is the chief source of that element. In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. In radioactive decay, there are lots of ways an unstable atom’s nucleus can transform to make it more stable. How Does Radium Decay.
From www.researchgate.net
(PDF) The environmental behavior of radium How Does Radium Decay That morphing process is called radioactive decay. One of the products of radium decay is radon, the heaviest noble gas; As radium decays, it releases radiation and forms decay products. Radium is not a stable element. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. To reach a lower, more stable energy. How Does Radium Decay.
From sciencedemonstrations.fas.harvard.edu
Radon's Progeny Decay Harvard Natural Sciences Lecture Demonstrations How Does Radium Decay Radium is not a stable element. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. This quickly. How Does Radium Decay.
From www.env.go.jp
Internal Exposure to Radon and Thoron through Inhalation [MOE] How Does Radium Decay Radium decays to produce radon gas. To reach a lower, more stable energy level, it releases energy in the form of radiation. The radioactive decay of pure radium releases an energy output of 28 w/kg; Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. In radioactive decay, there are lots of ways. How Does Radium Decay.
From www.epa.gov
Radioactive Decay Radiation Protection US EPA How Does Radium Decay To reach a lower, more stable energy level, it releases energy in the form of radiation. Radium decays to produce radon gas. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. In radioactive decay, there are lots of ways an unstable atom’s nucleus can transform to make it more stable — and. How Does Radium Decay.
From physicsopenlab.org
Some Alpha Spectra PhysicsOpenLab How Does Radium Decay This decay process is the chief source of that element. One of the products of radium decay is radon, the heaviest noble gas; In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. Like radium, many of these decay products also release radiation and form other. And decay reactions almost always involve. How Does Radium Decay.
From chemlin.org
Radium226 isotopic data and properties How Does Radium Decay To reach a lower, more stable energy level, it releases energy in the form of radiation. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. The radioactive decay of pure radium releases an energy output of 28 w/kg; This quickly increases to 160 w/kg within a few days through the establishment of. How Does Radium Decay.
From courses.lumenlearning.com
Radioactive Decay Chemistry How Does Radium Decay To reach a lower, more stable energy level, it releases energy in the form of radiation. In radioactive decay, there are lots of ways an unstable atom’s nucleus can transform to make it more stable — and smaller. Radium decays to produce radon gas. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the. How Does Radium Decay.
From www.radiation-dosimetry.org
What is Radioactive Decay Definition How Does Radium Decay Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. This quickly increases to 160 w/kg within a few days through the establishment of a secular. One of the products of radium decay is radon,. How Does Radium Decay.
From getkindledx.blogspot.com
Radioactive Decay Equation Of Radium 226 / Nuclides and Radioactive How Does Radium Decay This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. Radium is not a stable element. In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. The radioactive decay of pure radium releases an energy output of 28 w/kg; As radium decays, it releases radiation. How Does Radium Decay.
From www.youtube.com
Example Exponential Decay of Radium226 YouTube How Does Radium Decay Radium decays to produce radon gas. As radium decays, it releases radiation and forms decay products. In radioactive decay, there are lots of ways an unstable atom’s nucleus can transform to make it more stable — and smaller. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. In the natural environment, radium. How Does Radium Decay.
From www.epa.gov
Radioactive Decay US EPA How Does Radium Decay And decay reactions almost always involve giving off energy, radiation and more tiny particles. Like radium, many of these decay products also release radiation and form other. One of the products of radium decay is radon, the heaviest noble gas; This quickly increases to 160 w/kg within a few days through the establishment of a secular. To reach a lower,. How Does Radium Decay.
From radiumbymathunisa.blogspot.com
Radium, by Mathunisa Chandrakumar Radioactive Radium How Does Radium Decay That morphing process is called radioactive decay. This quickly increases to 160 w/kg within a few days through the establishment of a secular. Radium decays to produce radon gas. Radium is not a stable element. Radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. Like radium, many of these decay products also. How Does Radium Decay.
From www.researchgate.net
Decay chain and properties of Radium223. A) The predominant alpha (α How Does Radium Decay To reach a lower, more stable energy level, it releases energy in the form of radiation. Like radium, many of these decay products also release radiation and form other. One of the products of radium decay is radon, the heaviest noble gas; In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals.. How Does Radium Decay.
From www.sciencephoto.com
Radium decay Stock Image C026/2252 Science Photo Library How Does Radium Decay And decay reactions almost always involve giving off energy, radiation and more tiny particles. That morphing process is called radioactive decay. This decay process is the chief source of that element. Radium is not a stable element. One of the products of radium decay is radon, the heaviest noble gas; In radioactive decay, there are lots of ways an unstable. How Does Radium Decay.