Zinc Electron Affinity . Electron affinity can be either positive or. The ionisation energies of zinc are given below. Many species have anions that are not bound with respect to a free electron and the neutral species, in which case the calculations may give results. Ionisation energies and electron affinity. First ionization energy of zinc is 9.3941 ev. Electronegativity of zinc is 1.65. The electron affinity of zinc is 0 kj mol ‑1. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In chemistry and atomic physics, the electron. Electron affinity of zinc is — kj/mol. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. A representation of the atomic spectrum of zinc. The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of zinc is — kj/mol.
from www.mdpi.com
Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gaseous atom. First ionization energy of zinc is 9.3941 ev. Electron affinity of zinc is — kj/mol. The ionisation energies of zinc are given below. Ionisation energies and electron affinity. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. A representation of the atomic spectrum of zinc. The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. In chemistry and atomic physics, the electron. In chemistry and atomic physics, the electron affinity of an atom or molecule is.
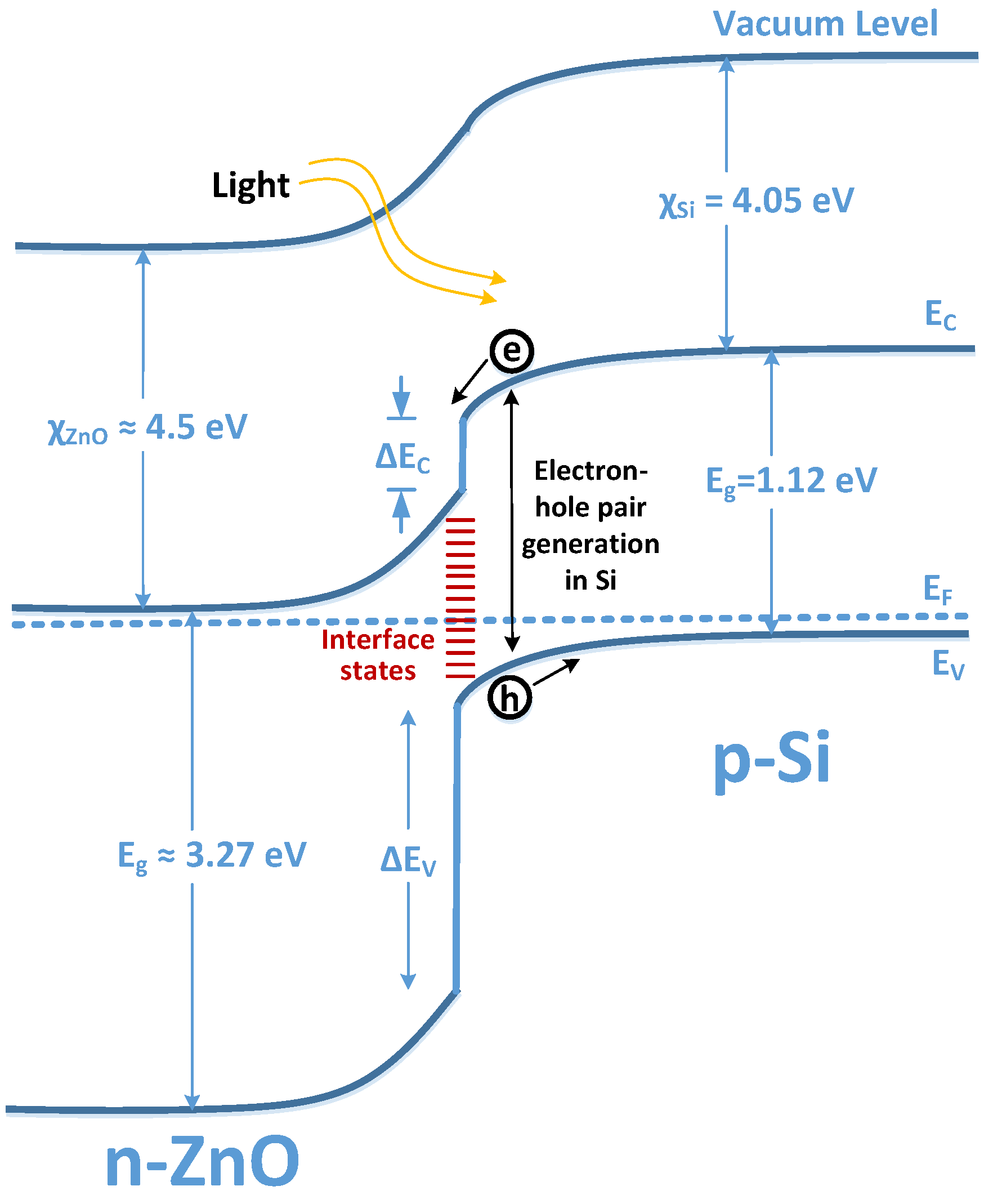
Electronics Free FullText Electron Affinity and Bandgap
Zinc Electron Affinity Electron affinity of zinc is — kj/mol. The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. A representation of the atomic spectrum of zinc. Ionisation energies and electron affinity. The ionisation energies of zinc are given below. The electron affinity of zinc is 0 kj mol ‑1. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Electron affinity can be either positive or. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gaseous atom. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of zinc is — kj/mol. In chemistry and atomic physics, the electron. Electronegativity of zinc is 1.65. First ionization energy of zinc is 9.3941 ev. Many species have anions that are not bound with respect to a free electron and the neutral species, in which case the calculations may give results. Electron affinity of zinc is — kj/mol.
From www.sciencephoto.com
Zinc electron configuration Stock Image C029/5029 Science Photo Zinc Electron Affinity 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. First ionization energy of zinc is 9.3941 ev. Many species have anions that are not bound with respect to a free electron and the neutral species, in which case the calculations may give. Zinc Electron Affinity.
From usermanualjingoism.z21.web.core.windows.net
Lewis Dot Diagram For Zinc Zinc Electron Affinity Ionisation energies and electron affinity. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gaseous atom. Many species have anions that are not bound with respect to a free electron and the neutral species, in which case the calculations may give results. A representation of the atomic spectrum of. Zinc Electron Affinity.
From www.mdpi.com
Electronics Free FullText Electron Affinity and Bandgap Zinc Electron Affinity The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. In chemistry and atomic physics, the electron affinity of an atom or molecule is. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. The ionisation energies of. Zinc Electron Affinity.
From stock.adobe.com
Zinc electron configuration and its propertiesvector illustration Zinc Electron Affinity The ionisation energies of zinc are given below. The electron affinity of zinc is 0 kj mol ‑1. Electronegativity of zinc is 1.65. Electron affinity can be either positive or. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. First ionization energy. Zinc Electron Affinity.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Affinity Ionisation energies and electron affinity. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. The ionisation energies of zinc are given below. Many species have anions that are not bound with respect to a free electron and the neutral species, in which. Zinc Electron Affinity.
From www.youtube.com
How to Find the Valence Electrons for Zinc (Zn) YouTube Zinc Electron Affinity Electron affinity of zinc is — kj/mol. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. The ionisation energies of zinc are given below. In chemistry and atomic physics, the electron. The electron affinity of zinc is 0 kj mol ‑1. Electron affinity of zinc is — kj/mol. The more. Zinc Electron Affinity.
From www.pinterest.com
Zinc Element Information, Facts, Properties, Trends,Uses, Comparison Zinc Electron Affinity First ionization energy of zinc is 9.3941 ev. Electronegativity of zinc is 1.65. The ionisation energies of zinc are given below. Electron affinity of zinc is — kj/mol. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gaseous atom. Ionisation energies and electron affinity. In chemistry and atomic physics,. Zinc Electron Affinity.
From www.youtube.com
zinc electronic configuration How to Write Zinc electronic Zinc Electron Affinity Ionisation energies and electron affinity. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. First ionization energy of zinc is 9.3941 ev. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. A. Zinc Electron Affinity.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Affinity Electron affinity can be either positive or. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of zinc is — kj/mol. The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. 119 rows electron affinity is the amount of energy. Zinc Electron Affinity.
From material-properties.org
Zinc Periodic Table and Atomic Properties Zinc Electron Affinity Electron affinity of zinc is — kj/mol. The ionisation energies of zinc are given below. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gaseous atom. Electron affinity of zinc is — kj/mol. In other words, when the electron is added to a neutral atom, the energy is either. Zinc Electron Affinity.
From pubchem.ncbi.nlm.nih.gov
Electron Affinity Periodic Table of Elements PubChem Zinc Electron Affinity Electron affinity can be either positive or. In chemistry and atomic physics, the electron affinity of an atom or molecule is. The ionisation energies of zinc are given below. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. The electron affinity of. Zinc Electron Affinity.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Zinc Electron Affinity Electron affinity can be either positive or. In chemistry and atomic physics, the electron. Ionisation energies and electron affinity. The electron affinity of zinc is 0 kj mol ‑1. The ionisation energies of zinc are given below. First ionization energy of zinc is 9.3941 ev. Many species have anions that are not bound with respect to a free electron and. Zinc Electron Affinity.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Affinity Ionisation energies and electron affinity. The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. A representation of the atomic spectrum of zinc. Electron affinity of zinc is — kj/mol. In chemistry and atomic physics, the electron. Electron affinity can be either positive or. In chemistry and. Zinc Electron Affinity.
From www.nuclear-power.com
Electron Affinity Zinc Electron Affinity Electron affinity of zinc is — kj/mol. Ionisation energies and electron affinity. The electron affinity of zinc is 0 kj mol ‑1. Electron affinity can be either positive or. In chemistry and atomic physics, the electron affinity of an atom or molecule is. First ionization energy of zinc is 9.3941 ev. Electron affinity of zinc is — kj/mol. Many species. Zinc Electron Affinity.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Affinity In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of zinc is — kj/mol. Electron affinity of zinc is — kj/mol. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gaseous atom. 119 rows electron affinity is the amount of energy change. Zinc Electron Affinity.
From www.mdpi.com
Electronics Free FullText Electron Affinity and Bandgap Zinc Electron Affinity In chemistry and atomic physics, the electron affinity of an atom or molecule is. The electron affinity of zinc is 0 kj mol ‑1. Electron affinity can be either positive or. Many species have anions that are not bound with respect to a free electron and the neutral species, in which case the calculations may give results. Electron affinity of. Zinc Electron Affinity.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Affinity Ionisation energies and electron affinity. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Electronegativity of zinc is 1.65. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gaseous atom. In. Zinc Electron Affinity.
From www.mdpi.com
Electronics Free FullText Electron Affinity and Bandgap Zinc Electron Affinity A representation of the atomic spectrum of zinc. Electron affinity of zinc is — kj/mol. Many species have anions that are not bound with respect to a free electron and the neutral species, in which case the calculations may give results. The electron affinity of zinc is 0 kj mol ‑1. 119 rows electron affinity is the amount of energy. Zinc Electron Affinity.
From www.youtube.com
Electron Configuration of Zinc Zn Lesson YouTube Zinc Electron Affinity Electronegativity of zinc is 1.65. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. First ionization energy of zinc is 9.3941 ev. The ionisation energies of zinc are given below. The more negative the electron affinity value, the higher the electron affinity. Zinc Electron Affinity.
From www.mdpi.com
Electronics Free FullText Electron Affinity and Bandgap Zinc Electron Affinity In chemistry and atomic physics, the electron. A representation of the atomic spectrum of zinc. Electron affinity of zinc is — kj/mol. The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. Many species have anions that are not bound with respect to a free electron and. Zinc Electron Affinity.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Affinity In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gaseous atom. In chemistry and atomic physics,. Zinc Electron Affinity.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Affinity The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. A representation of the atomic spectrum of zinc. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In other. Zinc Electron Affinity.
From www.alamy.com
Symbol and electron diagram for Zinc Stock Vector Image & Art Alamy Zinc Electron Affinity The ionisation energies of zinc are given below. The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. First ionization energy of zinc is 9.3941 ev. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gaseous. Zinc Electron Affinity.
From valenceelectrons.com
How to Write the Electron Configuration for Zinc (Zn)? Zinc Electron Affinity First ionization energy of zinc is 9.3941 ev. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. In chemistry and atomic physics, the electron affinity of an atom or molecule is. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in. Zinc Electron Affinity.
From www.alamy.com
Zn Zinc, Periodic Table of the Elements, Shell Structure of Zinc Zinc Electron Affinity The electron affinity of zinc is 0 kj mol ‑1. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Many species have anions that are not bound with respect to a free electron and the neutral. Zinc Electron Affinity.
From www.mdpi.com
Electronics Free FullText Electron Affinity and Bandgap Zinc Electron Affinity Electron affinity of zinc is — kj/mol. The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. The ionisation energies of zinc are given below. Electronegativity of zinc is 1.65. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added. Zinc Electron Affinity.
From www.nuclear-power.com
Zinc Electron Affinity Electronegativity Ionization Energy of Zinc Electron Affinity Electron affinity can be either positive or. First ionization energy of zinc is 9.3941 ev. In chemistry and atomic physics, the electron affinity of an atom or molecule is. The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. Electron affinity is a quantitative measurement of the. Zinc Electron Affinity.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Affinity Electron affinity of zinc is — kj/mol. First ionization energy of zinc is 9.3941 ev. Electron affinity of zinc is — kj/mol. A representation of the atomic spectrum of zinc. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gaseous atom. Electron affinity can be either positive or. Many. Zinc Electron Affinity.
From www.teachoo.com
[Chemistry Class 12] Account for (c) Zinc is comparatively soft metal Zinc Electron Affinity In chemistry and atomic physics, the electron. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Electron affinity can be either positive or. Electron affinity of zinc is — kj/mol. The more negative the electron affinity value, the higher the electron affinity. Zinc Electron Affinity.
From periodictable.me
Zinc Electron Configuration (Zn) with Orbital Diagram Zinc Electron Affinity Ionisation energies and electron affinity. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of zinc is — kj/mol. Electron affinity can be either positive or. The ionisation energies of zinc are given below. Electron affinity of zinc is — kj/mol. Electronegativity of zinc is 1.65. The more negative the electron affinity value,. Zinc Electron Affinity.
From animalia-life.club
Zinc Electron Configuration Zinc Electron Affinity A representation of the atomic spectrum of zinc. Electronegativity of zinc is 1.65. Electron affinity of zinc is — kj/mol. First ionization energy of zinc is 9.3941 ev. Ionisation energies and electron affinity. Many species have anions that are not bound with respect to a free electron and the neutral species, in which case the calculations may give results. The. Zinc Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Zinc Electron Affinity Electron affinity can be either positive or. Ionisation energies and electron affinity. First ionization energy of zinc is 9.3941 ev. The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is. Zinc Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Zinc Electron Affinity 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Electronegativity of zinc is 1.65. Electron affinity can be either positive or. The electron affinity of zinc is 0 kj mol ‑1. Many species have anions that are not bound with respect to. Zinc Electron Affinity.
From www.researchgate.net
Zincbinding affinity of AdcAs and PAR competition for zinc ions. (A Zinc Electron Affinity In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. Electron affinity of zinc is — kj/mol. Electronegativity of zinc is 1.65. Electron affinity can be either positive or. In chemistry and atomic physics, the electron. 119 rows electron affinity is the amount of energy change (δe) that occurs when an. Zinc Electron Affinity.
From www.researchgate.net
Zinc affinity of the two ZnT8 CTD variants. (A) Zinc binding to ZnT8cR Zinc Electron Affinity Many species have anions that are not bound with respect to a free electron and the neutral species, in which case the calculations may give results. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. The ionisation energies of zinc are given. Zinc Electron Affinity.