What Are Endo And Exothermic Reactions . The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This quiz will give you understanding of the basic properties and differences of exothermic and endothermic chemical reactions. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Photosynthesis is a good example of an endothermic. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Chemical reactions that release energy are called exothermic. The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from.
from vhmsscience.weebly.com
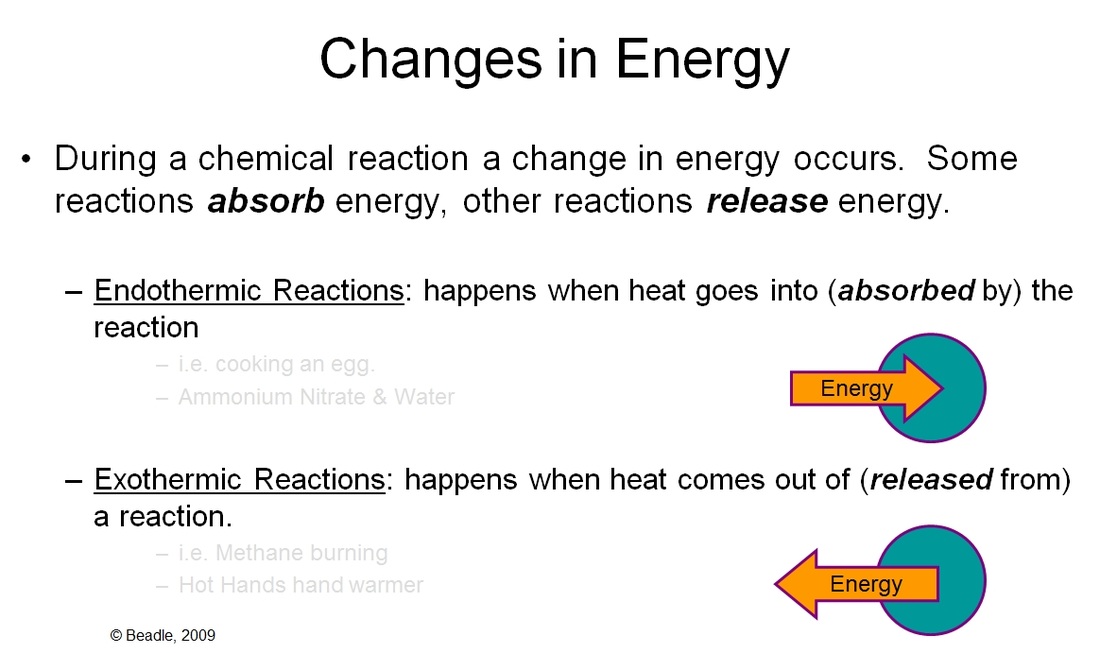
Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Chemical reactions that release energy are called exothermic. This quiz will give you understanding of the basic properties and differences of exothermic and endothermic chemical reactions. The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. Photosynthesis is a good example of an endothermic. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively.
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE
What Are Endo And Exothermic Reactions The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. This quiz will give you understanding of the basic properties and differences of exothermic and endothermic chemical reactions. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. Photosynthesis is a good example of an endothermic. The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding.
From gy.kimiq.com
In An Exothermic Reaction Energy Is Transferred From Energy Etfs What Are Endo And Exothermic Reactions The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Photosynthesis is a good example of an endothermic. The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. Chemical reactions that release energy are called exothermic. Endothermic and exothermic. What Are Endo And Exothermic Reactions.
From stock.adobe.com
Vecteur Stock Vector graphs or charts of endothermic and exothermic What Are Endo And Exothermic Reactions In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the. What Are Endo And Exothermic Reactions.
From mavink.com
Endothermic And Exothermic Reactions What Are Endo And Exothermic Reactions Photosynthesis is a good example of an endothermic. The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. The changes in energy that occur during a chemical reaction. What Are Endo And Exothermic Reactions.
From www.youtube.com
Exothermic and Endothermic Reactions and the Math Associated with Them What Are Endo And Exothermic Reactions Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. Chemical reactions that release energy are called exothermic. Photosynthesis is a good example of an endothermic. The main difference between exothermic and endothermic reactions is. What Are Endo And Exothermic Reactions.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions What Are Endo And Exothermic Reactions Chemical reactions that release energy are called exothermic. The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. Photosynthesis is a good example of an endothermic. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. Endothermic. What Are Endo And Exothermic Reactions.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE What Are Endo And Exothermic Reactions The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or. What Are Endo And Exothermic Reactions.
From www.youtube.com
Exothermic vs Endothermic Chemical Reactions YouTube What Are Endo And Exothermic Reactions Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. This quiz will give. What Are Endo And Exothermic Reactions.
From www.sexiezpix.com
What Are Endothermic Reactions With Examples Video SexiezPix Porn What Are Endo And Exothermic Reactions Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This quiz will give you understanding. What Are Endo And Exothermic Reactions.
From khatiar1955a.altervista.org
The Difference Between Endothermic and Exothermic Reactions What Are Endo And Exothermic Reactions Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. This quiz will give you understanding of the basic properties and differences of exothermic and endothermic chemical reactions. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Photosynthesis is a good example of an endothermic.. What Are Endo And Exothermic Reactions.
From stock.adobe.com
Endothermic Vs Exothermic Reactions Infographic Diagram showing a What Are Endo And Exothermic Reactions The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. Chemical reactions that release energy are called exothermic. The main difference between exothermic and endothermic reactions is that. What Are Endo And Exothermic Reactions.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples What Are Endo And Exothermic Reactions In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. The changes in energy. What Are Endo And Exothermic Reactions.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry What Are Endo And Exothermic Reactions Chemical reactions that release energy are called exothermic. The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. In exothermic reactions, more energy is released when the bonds. What Are Endo And Exothermic Reactions.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE What Are Endo And Exothermic Reactions In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Chemical reactions that release energy are called exothermic. Endothermic and exothermic reactions can be thought of as having. What Are Endo And Exothermic Reactions.
From online-learning-college.com
Exothermic and endothermic reactions Key differences What Are Endo And Exothermic Reactions The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. Photosynthesis is a good example of an endothermic. Endothermic and exothermic reactions can be thought of as. What Are Endo And Exothermic Reactions.
From www.slideserve.com
PPT Endothermic and Exothermic Reactions PowerPoint Presentation What Are Endo And Exothermic Reactions Chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. Photosynthesis is a good example of an endothermic. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Endothermic and exothermic reactions can be thought of as. What Are Endo And Exothermic Reactions.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE What Are Endo And Exothermic Reactions The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Chemical reactions that release energy are called exothermic. Photosynthesis is a good example of an endothermic. This quiz will give you understanding of the basic properties and. What Are Endo And Exothermic Reactions.
From www.youtube.com
Endothermic Vs. Exothermic Reaction Graphs YouTube What Are Endo And Exothermic Reactions Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. The changes in energy that occur during a chemical reaction can be seen by examining the changes. What Are Endo And Exothermic Reactions.
From 5differencebetween.com
5 Difference Between Endothermic and Exothermic Reaction Endothermic What Are Endo And Exothermic Reactions The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. Photosynthesis is a good example of an endothermic. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Chemical reactions that release energy are called exothermic. Endothermic and exothermic. What Are Endo And Exothermic Reactions.
From sciencenotes.org
Endothermic Reactions Definition and Examples What Are Endo And Exothermic Reactions This quiz will give you understanding of the basic properties and differences of exothermic and endothermic chemical reactions. Photosynthesis is a good example of an endothermic. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. Endothermic and exothermic reactions can be thought of as having energy as. What Are Endo And Exothermic Reactions.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition What Are Endo And Exothermic Reactions Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. This quiz will give you understanding of the basic properties and differences of exothermic and endothermic chemical reactions. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Chemical reactions that release energy are called exothermic.. What Are Endo And Exothermic Reactions.
From www.youtube.com
Endothermic and Exothermic Reactions With Potential Energy Diagrams What Are Endo And Exothermic Reactions Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Chemical reactions that release. What Are Endo And Exothermic Reactions.
From www.vrogue.co
Identify The Endothermic And Exothermic Reaction vrogue.co What Are Endo And Exothermic Reactions The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. Chemical reactions that release energy are called exothermic. Photosynthesis is a good example of an endothermic. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Endothermic and exothermic. What Are Endo And Exothermic Reactions.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching What Are Endo And Exothermic Reactions Photosynthesis is a good example of an endothermic. Chemical reactions that release energy are called exothermic. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This quiz will give you understanding of the basic properties and differences of exothermic and endothermic chemical reactions. The main difference between exothermic and. What Are Endo And Exothermic Reactions.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give What Are Endo And Exothermic Reactions Photosynthesis is a good example of an endothermic. Chemical reactions that release energy are called exothermic. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Endothermic and exothermic reactions can be thought of as having energy. What Are Endo And Exothermic Reactions.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give What Are Endo And Exothermic Reactions Photosynthesis is a good example of an endothermic. This quiz will give you understanding of the basic properties and differences of exothermic and endothermic chemical reactions. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. The changes in energy that occur during a chemical reaction can be. What Are Endo And Exothermic Reactions.
From kunduz.com
Exothermic Reaction Definition, Properties, Examples, Exothermic and What Are Endo And Exothermic Reactions Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. Photosynthesis is a good example of an endothermic. This quiz will give you understanding of the basic properties and differences of exothermic and endothermic chemical reactions. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the. What Are Endo And Exothermic Reactions.
From www.youtube.com
Endothermic and exothermic reactions. Enthalpy YouTube What Are Endo And Exothermic Reactions Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Photosynthesis is a good example of. What Are Endo And Exothermic Reactions.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE What Are Endo And Exothermic Reactions The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Photosynthesis is a good example of an endothermic. The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. Endothermic and exothermic reactions are chemical reactions that absorb and release. What Are Endo And Exothermic Reactions.
From www.pinterest.co.uk
Endothermic and Exothermic Reactions Lab ⋆ Exothermic What Are Endo And Exothermic Reactions Chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. The main difference between exothermic and endothermic reactions is that. What Are Endo And Exothermic Reactions.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk What Are Endo And Exothermic Reactions Chemical reactions that release energy are called exothermic. Photosynthesis is a good example of an endothermic. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This quiz will give you understanding of the basic properties and differences of exothermic and endothermic chemical reactions. In exothermic reactions, more energy is. What Are Endo And Exothermic Reactions.
From www.slideserve.com
PPT Energy Changes PowerPoint Presentation, free download ID3470966 What Are Endo And Exothermic Reactions Chemical reactions that release energy are called exothermic. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction. What Are Endo And Exothermic Reactions.
From www.youtube.com
Chemical Reaction and equation (Exothermic and Endothermic Reaction What Are Endo And Exothermic Reactions Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. The changes in energy. What Are Endo And Exothermic Reactions.
From question.pandai.org
Endothermic and exothermic reactions What Are Endo And Exothermic Reactions Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds. The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the. What Are Endo And Exothermic Reactions.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk What Are Endo And Exothermic Reactions The main difference between exothermic and endothermic reactions is that an endothermic reaction absorbs energy in the form of heat from. Chemical reactions that release energy are called exothermic. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. This quiz will give you understanding of the basic properties. What Are Endo And Exothermic Reactions.
From eduinput.com
Difference Between Endothermic And Exothermic Reactions What Are Endo And Exothermic Reactions Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. This quiz will give you understanding. What Are Endo And Exothermic Reactions.