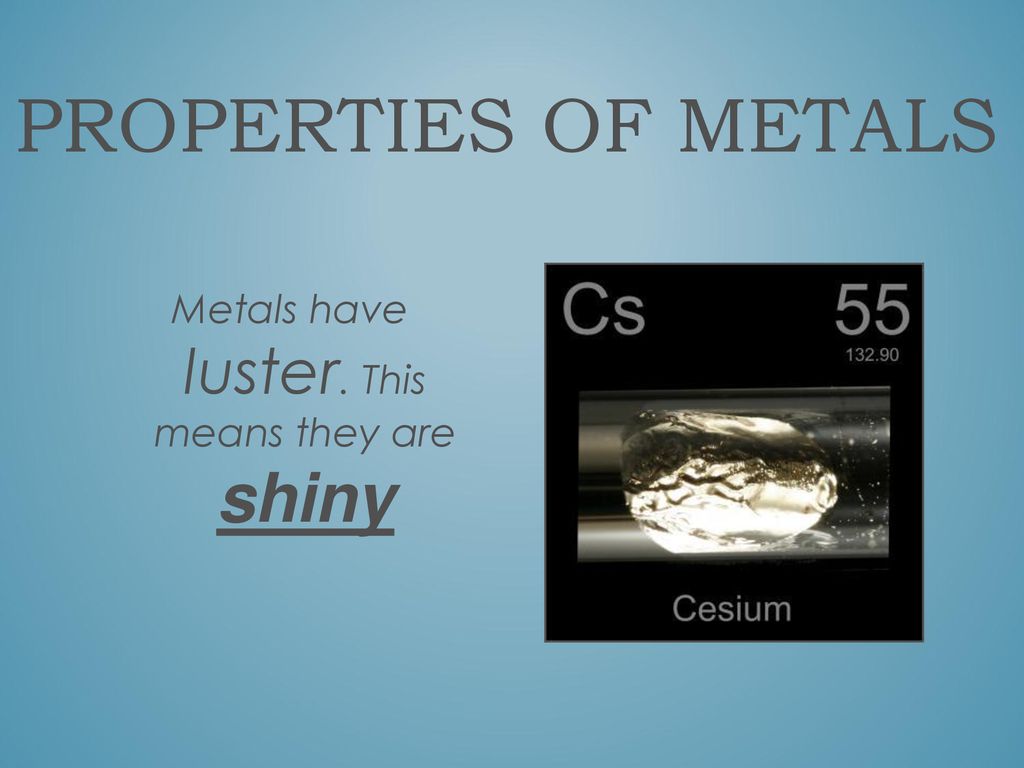
Why Do Metals Appear Shiny . What makes copper and gold have different colors? Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. The outermost electrons of a metal atom are loosely bound. Many different kinds of metal are shiny. Why do most metals (iron, tin, aluminum, lead, zinc, tungsten, nickel, etc.) appear silver or gray? Some regions on the metal become relative. When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. Metals have a shiny appearance due to their unique atomic structure. Metals are shiny because metals contain free electrons that vibrate when they come in contact with light. This phenomenon, known as specular reflection, occurs when light rays bounce off the surface without scattering or being. Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. Why do metals have a shiny appearance?
from slideplayer.com
Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. This phenomenon, known as specular reflection, occurs when light rays bounce off the surface without scattering or being. Metals are shiny because metals contain free electrons that vibrate when they come in contact with light. The outermost electrons of a metal atom are loosely bound. Why do most metals (iron, tin, aluminum, lead, zinc, tungsten, nickel, etc.) appear silver or gray? When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. What makes copper and gold have different colors? Metals have a shiny appearance due to their unique atomic structure. Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. Some regions on the metal become relative.
The Periodic Table of Elements ppt download
Why Do Metals Appear Shiny When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. This phenomenon, known as specular reflection, occurs when light rays bounce off the surface without scattering or being. Metals have a shiny appearance due to their unique atomic structure. What makes copper and gold have different colors? Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. Why do metals have a shiny appearance? Some regions on the metal become relative. The outermost electrons of a metal atom are loosely bound. Metals are shiny because metals contain free electrons that vibrate when they come in contact with light. Why do most metals (iron, tin, aluminum, lead, zinc, tungsten, nickel, etc.) appear silver or gray? When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. Many different kinds of metal are shiny.
From engineeringlearner.com
Types of Metals and Their Uses [with Pictures] Engineering Learner Why Do Metals Appear Shiny The outermost electrons of a metal atom are loosely bound. Some regions on the metal become relative. This phenomenon, known as specular reflection, occurs when light rays bounce off the surface without scattering or being. Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. What makes copper and. Why Do Metals Appear Shiny.
From slideplayer.com
Periodic Table of Elements ppt download Why Do Metals Appear Shiny Some regions on the metal become relative. Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. Metals have a shiny appearance due to their unique atomic structure. Many different kinds of metal are shiny. Metals have a shiny appearance because of their unique atomic structure, which allows them. Why Do Metals Appear Shiny.
From www.chegg.com
Solved [Chapter 21] Why do metals appear shiny? Egap > 3.1 Why Do Metals Appear Shiny Metals have a shiny appearance due to their unique atomic structure. When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. Why do metals have a shiny appearance? Metals have a shiny. Why Do Metals Appear Shiny.
From www.chemistrylearner.com
Metalloids Chemistry Learner Why Do Metals Appear Shiny Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. Many different kinds of metal are shiny. When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. Metals are shiny because metals contain free electrons that vibrate when they come in contact. Why Do Metals Appear Shiny.
From metalprofy.com
Why Do Metals Have High Melting Points? MetalProfy Why Do Metals Appear Shiny Metals are shiny because metals contain free electrons that vibrate when they come in contact with light. The outermost electrons of a metal atom are loosely bound. Why do metals have a shiny appearance? This phenomenon, known as specular reflection, occurs when light rays bounce off the surface without scattering or being. Some regions on the metal become relative. When. Why Do Metals Appear Shiny.
From slideplayer.com
The Periodic Table of Elements ppt download Why Do Metals Appear Shiny Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. Metals are shiny because metals contain. Why Do Metals Appear Shiny.
From sites.wustl.edu
Metal, Iron, & Nickel Some Meteorite Information Washington Why Do Metals Appear Shiny Some regions on the metal become relative. What makes copper and gold have different colors? When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. Many different kinds of metal are shiny. Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. Metals have a shiny appearance. Why Do Metals Appear Shiny.
From en.ppt-online.org
Metals online presentation Why Do Metals Appear Shiny Metals are shiny because metals contain free electrons that vibrate when they come in contact with light. Why do metals have a shiny appearance? Metals have a shiny appearance due to their unique atomic structure. Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. Some regions on the. Why Do Metals Appear Shiny.
From engineerfix.com
Why Do Metals Bend? Causes, How They Bend And Some FAQs Why Do Metals Appear Shiny This phenomenon, known as specular reflection, occurs when light rays bounce off the surface without scattering or being. Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. Metals are shiny because metals contain free electrons that vibrate when they come in contact with light. Many different kinds of. Why Do Metals Appear Shiny.
From www.difference101.com
Metals vs. Nonmetals vs. Metalloids 5 Key Differences, Pros & Cons Why Do Metals Appear Shiny Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. Many different kinds of metal are shiny. Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. When a beam of light is incident on a metal surface, it polarizes the electron cloud,. Why Do Metals Appear Shiny.
From edurev.in
Why metals are lusture? EduRev Class 10 Question Why Do Metals Appear Shiny Many different kinds of metal are shiny. When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. Why do most metals (iron, tin, aluminum, lead, zinc, tungsten, nickel, etc.) appear silver or gray? What makes copper and gold have different colors? Why do metals have a shiny appearance? The outermost electrons of a. Why Do Metals Appear Shiny.
From slideplayer.com
Elements and Compounds Chapter 3 ppt download Why Do Metals Appear Shiny Many different kinds of metal are shiny. Why do metals have a shiny appearance? Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. Why do most metals (iron, tin, aluminum, lead, zinc, tungsten, nickel, etc.) appear silver or gray? Metals are shiny because metals contain free electrons that. Why Do Metals Appear Shiny.
From behance.net
Material Studies Metals on Behance Why Do Metals Appear Shiny Metals have a shiny appearance due to their unique atomic structure. Some regions on the metal become relative. Many different kinds of metal are shiny. When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. Gold is a metal which stays shiny for a long time because it does not react much chemically. Why Do Metals Appear Shiny.
From www.youtube.com
Metals are said to be shiny. Why do metals generally appear to be d Why Do Metals Appear Shiny Metals have a shiny appearance due to their unique atomic structure. Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. Metals are shiny because metals contain free electrons that vibrate when they come in contact with light. When a beam of light is incident on a metal surface,. Why Do Metals Appear Shiny.
From slideplayer.com
OPTICAL PROPERTIES K L University Department of Physics. ppt download Why Do Metals Appear Shiny Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. The outermost electrons of a metal atom are loosely bound. Some regions on the metal become relative. Metals are shiny because metals contain free electrons that vibrate when they come in contact with light. This phenomenon, known as specular reflection, occurs when light rays. Why Do Metals Appear Shiny.
From www.youtube.com
Why do metals have lustrous surface? YouTube Why Do Metals Appear Shiny Why do most metals (iron, tin, aluminum, lead, zinc, tungsten, nickel, etc.) appear silver or gray? Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. Many different kinds of metal are shiny. Gold is a metal which stays shiny for a long time because it does not react much chemically with the air.. Why Do Metals Appear Shiny.
From treasurepursuits.com
So You Found a Shiny Black Rock 6 Things it Might Be Why Do Metals Appear Shiny What makes copper and gold have different colors? Metals have a shiny appearance due to their unique atomic structure. Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. Why do most metals (iron, tin, aluminum, lead, zinc, tungsten, nickel, etc.) appear silver or gray? Some regions on the metal become relative. Many different. Why Do Metals Appear Shiny.
From slidetodoc.com
Periodic Table of Elements Metals Properties of Metals Why Do Metals Appear Shiny Metals have a shiny appearance due to their unique atomic structure. This phenomenon, known as specular reflection, occurs when light rays bounce off the surface without scattering or being. Why do most metals (iron, tin, aluminum, lead, zinc, tungsten, nickel, etc.) appear silver or gray? Metals have a shiny appearance because of their unique atomic structure, which allows them to. Why Do Metals Appear Shiny.
From simpleeducation.biz
Why Are Metals Shiny? Simple Education Why Do Metals Appear Shiny Some regions on the metal become relative. Metals are shiny because metals contain free electrons that vibrate when they come in contact with light. Why do metals have a shiny appearance? Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. When a beam of light is incident on. Why Do Metals Appear Shiny.
From www.simplechemconcepts.com
OLevel and IP Pure Chemistry Reactivity Series of Metals Why Do Metals Appear Shiny The outermost electrons of a metal atom are loosely bound. Some regions on the metal become relative. Why do metals have a shiny appearance? Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. Gold is a metal which stays shiny for a long time because it does not react much chemically with the. Why Do Metals Appear Shiny.
From engineerfix.com
Why Do Metals Bend? Causes, How They Bend And Some FAQs Why Do Metals Appear Shiny Some regions on the metal become relative. Many different kinds of metal are shiny. Why do metals have a shiny appearance? The outermost electrons of a metal atom are loosely bound. What makes copper and gold have different colors? Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. Why do most metals (iron,. Why Do Metals Appear Shiny.
From msestudent.com
Why Do Metals Conduct Electricity? Materials Science & Engineering Why Do Metals Appear Shiny This phenomenon, known as specular reflection, occurs when light rays bounce off the surface without scattering or being. The outermost electrons of a metal atom are loosely bound. Why do metals have a shiny appearance? Some regions on the metal become relative. Many different kinds of metal are shiny. What makes copper and gold have different colors? When a beam. Why Do Metals Appear Shiny.
From slideplayer.com
Chapter 12 The Periodic Table. ppt download Why Do Metals Appear Shiny Metals are shiny because metals contain free electrons that vibrate when they come in contact with light. Many different kinds of metal are shiny. Some regions on the metal become relative. Why do metals have a shiny appearance? When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. Metals have a shiny appearance. Why Do Metals Appear Shiny.
From www.slideserve.com
PPT Light PowerPoint Presentation, free download ID1969007 Why Do Metals Appear Shiny Metals are shiny because metals contain free electrons that vibrate when they come in contact with light. This phenomenon, known as specular reflection, occurs when light rays bounce off the surface without scattering or being. Many different kinds of metal are shiny. Gold is a metal which stays shiny for a long time because it does not react much chemically. Why Do Metals Appear Shiny.
From mungfali.com
Periodic Table Of Elements Alkali Metals Why Do Metals Appear Shiny This phenomenon, known as specular reflection, occurs when light rays bounce off the surface without scattering or being. When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. Some regions on the. Why Do Metals Appear Shiny.
From k24k.wordpress.com
Why are metals shiny? K24K Why Do Metals Appear Shiny Why do most metals (iron, tin, aluminum, lead, zinc, tungsten, nickel, etc.) appear silver or gray? The outermost electrons of a metal atom are loosely bound. When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. Some regions on the metal become relative. What makes copper and gold have different colors? Gold is. Why Do Metals Appear Shiny.
From www.youtube.com
Metals are said to be shiny. Why do metals generally appear to be dull Why Do Metals Appear Shiny Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. The outermost electrons of a metal atom are loosely bound. Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. Why do most metals (iron, tin, aluminum, lead, zinc, tungsten, nickel, etc.) appear. Why Do Metals Appear Shiny.
From www.youtube.com
Why are alloys stronger than pure metals? YouTube Why Do Metals Appear Shiny Why do metals have a shiny appearance? The outermost electrons of a metal atom are loosely bound. When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. What makes copper and gold have different colors? Metals have a shiny appearance due to their unique atomic structure. Gold is a metal which stays shiny. Why Do Metals Appear Shiny.
From www.youtube.com
Why d block metals have higher melting points than s block metals Why Do Metals Appear Shiny Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. The outermost electrons of a metal atom are loosely bound. Metals have a shiny appearance due to their unique atomic structure. Some regions on the metal become relative. Why do most metals (iron, tin, aluminum, lead, zinc, tungsten, nickel, etc.) appear silver or gray?. Why Do Metals Appear Shiny.
From www.meritnation.com
Metals are said to be shiny Why do metals generally appear to be dull Why Do Metals Appear Shiny Some regions on the metal become relative. Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. Metals have a shiny appearance due to their unique atomic structure. When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. Why do most metals (iron, tin, aluminum, lead, zinc,. Why Do Metals Appear Shiny.
From www.tes.com
Metals Vs NonMetals Teaching Resources Why Do Metals Appear Shiny This phenomenon, known as specular reflection, occurs when light rays bounce off the surface without scattering or being. Metals are shiny because metals contain free electrons that vibrate when they come in contact with light. Some regions on the metal become relative. When a beam of light is incident on a metal surface, it polarizes the electron cloud, i.e. Why. Why Do Metals Appear Shiny.
From www.youtube.com
Ask a Chemist Why do metals look metallic? (and other questions) YouTube Why Do Metals Appear Shiny Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. Many different kinds of metal are shiny. What makes copper and gold have different colors? Why do metals have a shiny appearance? Metals are shiny because metals contain free electrons that vibrate when they come in contact with light.. Why Do Metals Appear Shiny.
From sciencenotes.org
Metals vs Nonmetals Why Do Metals Appear Shiny Metals have a shiny appearance due to their unique atomic structure. Why do most metals (iron, tin, aluminum, lead, zinc, tungsten, nickel, etc.) appear silver or gray? Metals have a shiny appearance because of their unique atomic structure, which allows them to reflect. Gold is a metal which stays shiny for a long time because it does not react much. Why Do Metals Appear Shiny.
From www.dreamstime.com
Shiny metals stock photo. Image of forming, cylinder, grind 3618308 Why Do Metals Appear Shiny The outermost electrons of a metal atom are loosely bound. Metals are shiny because metals contain free electrons that vibrate when they come in contact with light. Why do metals have a shiny appearance? This phenomenon, known as specular reflection, occurs when light rays bounce off the surface without scattering or being. Some regions on the metal become relative. Metals. Why Do Metals Appear Shiny.
From studylib.net
Metals Why Do Metals Appear Shiny This phenomenon, known as specular reflection, occurs when light rays bounce off the surface without scattering or being. Metals have a shiny appearance due to their unique atomic structure. Gold is a metal which stays shiny for a long time because it does not react much chemically with the air. Why do most metals (iron, tin, aluminum, lead, zinc, tungsten,. Why Do Metals Appear Shiny.