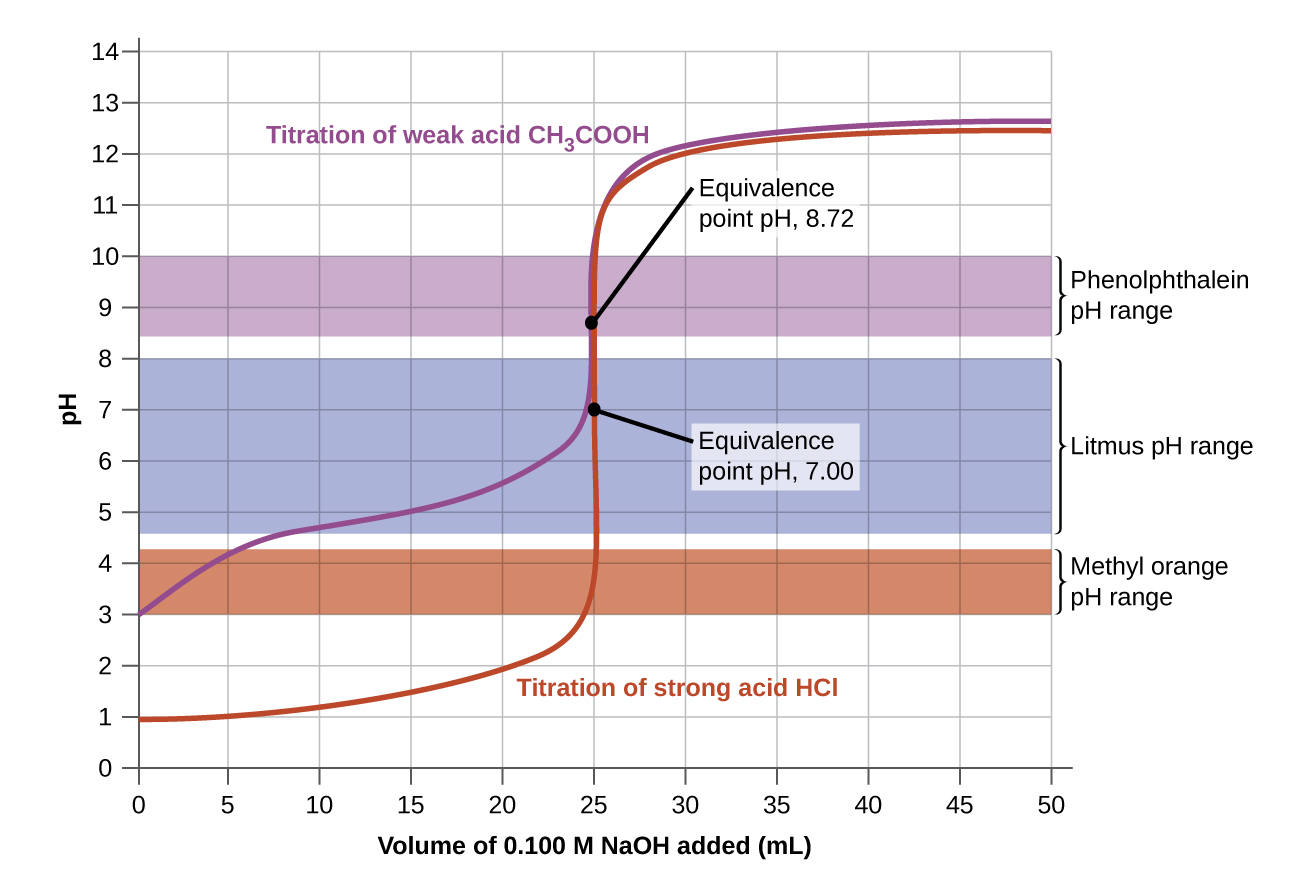
Titration The End Point . Titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Using a ph probe and using a colour indicator. Titrations with a ph probe. During the process, two important stages known as endpoint and equivalence point are reached. A point of equivalence in a titration refers to a. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The red arrow shows the location of the titration’s end point.
from philschatz.com
Titrations with a ph probe. The red arrow shows the location of the titration’s end point. A point of equivalence in a titration refers to a. During the process, two important stages known as endpoint and equivalence point are reached. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Using a ph probe and using a colour indicator. Titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified. For a ph titration (between an acid and a base), there are two common ways to find the endpoint:
AcidBase Titrations · Chemistry
Titration The End Point The red arrow shows the location of the titration’s end point. Titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified. A point of equivalence in a titration refers to a. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The red arrow shows the location of the titration’s end point. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. During the process, two important stages known as endpoint and equivalence point are reached. Titrations with a ph probe.
From philschatz.com
AcidBase Titrations · Chemistry Titration The End Point During the process, two important stages known as endpoint and equivalence point are reached. Titrations with a ph probe. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: In the overview to this chapter we noted that a titration’s end point. Titration The End Point.
From www.slideserve.com
PPT Chapter 8 Acids and Bases PowerPoint Presentation, free download Titration The End Point A point of equivalence in a titration refers to a. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Using a ph probe and using a colour indicator. Titrations with a ph probe. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added. Titration The End Point.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration The End Point Titrations with a ph probe. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified. During the process, two important stages known as endpoint and equivalence point. Titration The End Point.
From www.learnsci.com
LearnSci LabSim Titration End Point Titration The End Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titrations with a ph probe. Using a ph probe. Titration The End Point.
From kamora-kjuarez.blogspot.com
What Is an End Point in Titration Titration The End Point Titrations with a ph probe. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A point of equivalence in a titration refers to a. During the process, two important stages known as endpoint and equivalence point are. Titration The End Point.
From ar.inspiredpencil.com
Endpoint Titration Titration The End Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titrations with a ph probe. A point of equivalence. Titration The End Point.
From www.researchgate.net
How to determine the end point of EDTA titration? Titration The End Point For a ph titration (between an acid and a base), there are two common ways to find the endpoint: During the process, two important stages known as endpoint and equivalence point are reached. The red arrow shows the location of the titration’s end point. In the overview to this chapter we noted that a titration’s end point should coincide with. Titration The End Point.
From www.coursehero.com
[Solved] For the graphic determination of a titration end point the Titration The End Point In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The red arrow shows the location of the titration’s end point. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point. Titration The End Point.
From www.differencebetween.com
Difference Between AcidBase Titration and Redox Titration Compare Titration The End Point A point of equivalence in a titration refers to a. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titrations with a ph probe. During the process, two important stages known as endpoint and equivalence point are reached. Using a ph probe and using a colour indicator. Titration (also known. Titration The End Point.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration The End Point Using a ph probe and using a colour indicator. Titrations with a ph probe. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of. Titration The End Point.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Titration The End Point Titrations with a ph probe. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A point of equivalence in a titration refers to a. During the process, two important stages known as endpoint and equivalence point are. Titration The End Point.
From theedge.com.hk
Chemistry How To Titration The Edge Titration The End Point Titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified. A point of equivalence in a titration refers to a. During the process, two important stages known as endpoint and equivalence point are reached. A titration is a volumetric technique in which a solution of one. Titration The End Point.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration The End Point A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator.. Titration The End Point.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration The End Point The red arrow shows the location of the titration’s end point. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: During the process, two important stages known as endpoint and equivalence point are reached. A point of equivalence in a titration. Titration The End Point.
From schoolbag.info
Titration and Buffers Acids and Bases Titration The End Point A point of equivalence in a titration refers to a. Titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titrations with a ph probe. A titration. Titration The End Point.
From www.slideserve.com
PPT CH 103 ACIDBASE TITRATIONS PowerPoint Presentation, free Titration The End Point In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titrations with a ph probe. The red arrow shows the location of the titration’s end point. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titration (also known as titrimetry[1] and. Titration The End Point.
From www.animalia-life.club
Endpoint Titration Titration The End Point Titrations with a ph probe. Using a ph probe and using a colour indicator. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A point of equivalence in a titration refers. Titration The End Point.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Titration The End Point The red arrow shows the location of the titration’s end point. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A point of equivalence in a titration refers to a. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titration. Titration The End Point.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder Titration The End Point Using a ph probe and using a colour indicator. During the process, two important stages known as endpoint and equivalence point are reached. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titrations with a ph probe. For a ph titration (between an acid and a base), there are two. Titration The End Point.
From www.researchgate.net
The relation between alkalinity end point and pH titration curve. End Titration The End Point Titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The red arrow shows the location of the titration’s end point. A titration is a volumetric technique. Titration The End Point.
From www.animalia-life.club
Endpoint Titration Titration The End Point For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titrations with a ph probe. Titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified. Using a ph probe and using a colour indicator. In the overview. Titration The End Point.
From slideplayer.com
Titration Colour Changes ppt download Titration The End Point A point of equivalence in a titration refers to a. Using a ph probe and using a colour indicator. During the process, two important stages known as endpoint and equivalence point are reached. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The red arrow shows the location of the. Titration The End Point.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation ID431911 Titration The End Point A point of equivalence in a titration refers to a. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Using a ph probe and using a colour indicator. During the process, two important stages known as endpoint. Titration The End Point.
From slideplayer.com
Titrations!. ppt download Titration The End Point The red arrow shows the location of the titration’s end point. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point. Titration The End Point.
From www.slideserve.com
PPT CHAPTER 5 Volumetric Analysis PowerPoint Presentation, free Titration The End Point In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The red arrow shows the location of the titration’s end point. Titration (also known as titrimetry[1] and volumetric analysis) is a common. Titration The End Point.
From www.animalia-life.club
Endpoint Titration Titration The End Point Titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified. During the process, two important stages known as endpoint and equivalence point are reached. The red arrow shows the location of the titration’s end point. For a ph titration (between an acid and a base), there. Titration The End Point.
From edurev.in
End Point And Equivalence Point Mole Concept Chemistry Notes EduRev Titration The End Point A point of equivalence in a titration refers to a. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A titration is a volumetric technique in which a solution of one. Titration The End Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration The End Point A point of equivalence in a titration refers to a. Titrations with a ph probe. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. For a ph titration (between an acid and a base), there are two. Titration The End Point.
From fineartamerica.com
End Point Of An Iodine Titration. 3 Of 5. Photograph by Martyn F Titration The End Point During the process, two important stages known as endpoint and equivalence point are reached. The red arrow shows the location of the titration’s end point. Using a ph probe and using a colour indicator. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte). Titration The End Point.
From socratic.org
The "pH" at onehalf the equivalence point in an acidbase titration Titration The End Point The red arrow shows the location of the titration’s end point. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point. Titration The End Point.
From kamora-kjuarez.blogspot.com
What Is an End Point in Titration Titration The End Point Titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The red arrow shows the location of the titration’s end point. Titrations with a ph probe. During. Titration The End Point.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration The End Point Titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: During the process, two important stages known as endpoint and equivalence point are reached. The red arrow. Titration The End Point.
From www.animalia-life.club
Endpoint Titration Titration The End Point The red arrow shows the location of the titration’s end point. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titrations with a ph probe. Using a ph probe and using a colour indicator. During the process, two important stages known as endpoint and equivalence point are reached. A titration. Titration The End Point.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration The End Point During the process, two important stages known as endpoint and equivalence point are reached. The red arrow shows the location of the titration’s end point. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: In the overview to this chapter we noted that a titration’s end point should coincide with. Titration The End Point.
From www.animalia-life.club
Endpoint Titration Titration The End Point For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titration (also known as titrimetry[1] and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified. Using a ph probe and using a colour indicator. During the process, two important stages known as. Titration The End Point.