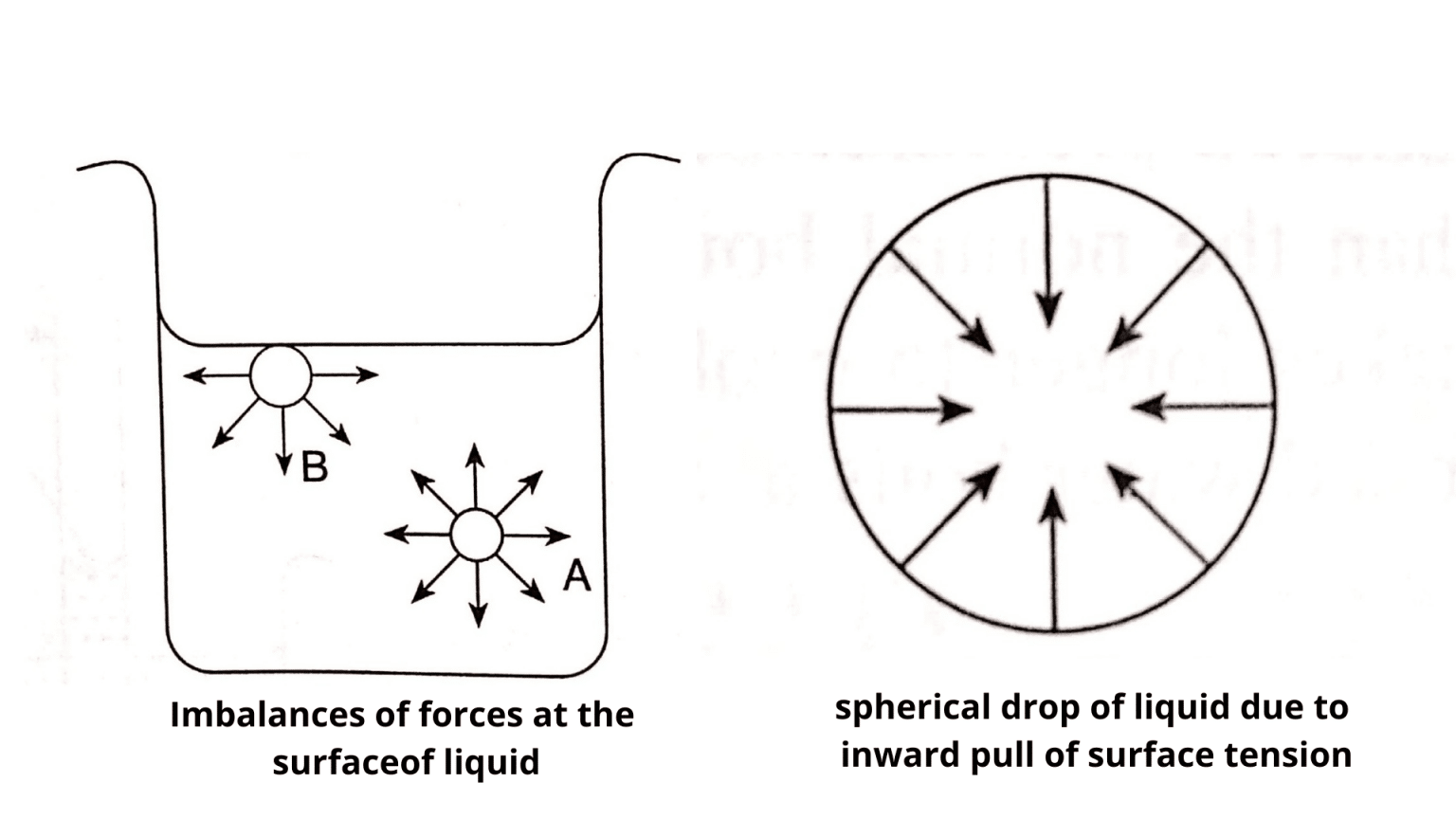
Surface Tension Summary . A fluid surface has a tendency to occupy as little. This phenomenon can be observed in the nearly. On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to calculate it using the formula f/l. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Quantifies surface tension as the tension force exerted per unit length, represented as: Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface.
from chemistnotes.com
A fluid surface has a tendency to occupy as little. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. Quantifies surface tension as the tension force exerted per unit length, represented as: This phenomenon can be observed in the nearly. On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to calculate it using the formula f/l. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane.
Surface Tension Definition, Units, Epic Examples, Effects, and
Surface Tension Summary Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to calculate it using the formula f/l. On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Quantifies surface tension as the tension force exerted per unit length, represented as: This phenomenon can be observed in the nearly. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. A fluid surface has a tendency to occupy as little.
From www.istockphoto.com
140+ Acting Surface Stock Photos, Pictures & RoyaltyFree Images iStock Surface Tension Summary On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Quantifies surface tension as the tension force exerted per unit length, represented as: Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This phenomenon can be observed in the. Surface Tension Summary.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Surface Tension Summary Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to calculate it using the formula f/l. Quantifies surface tension as the tension force exerted per unit length, represented as:. Surface Tension Summary.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Surface Tension Summary On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. A fluid surface has a tendency to occupy as little. Quantifies surface tension as the tension force exerted per unit length, represented as: Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area. Surface Tension Summary.
From www.numerade.com
SOLVED Find the dimensional formula of surface tension S. The formula Surface Tension Summary Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to. Surface Tension Summary.
From tfiglobalnews.com
The Relation Between Surface Tension and Surface Energy TFIGlobal Surface Tension Summary Quantifies surface tension as the tension force exerted per unit length, represented as: On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to calculate it using the formula f/l.. Surface Tension Summary.
From byjus.com
Explain the surface tension phenomenon with examples. Surface Tension Summary Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to calculate it using the formula f/l. On account of the cohesive forces between the molecules of a liquid the. Surface Tension Summary.
From eduinput.com
Surface TensionDefinition, Examples, Causes, And Measurement Surface Tension Summary Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This phenomenon can be observed in the nearly. On account of the cohesive forces between the molecules of a liquid. Surface Tension Summary.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Surface Tension Summary Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to. Surface Tension Summary.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Surface Tension Summary Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area. Surface Tension Summary.
From www.vecteezy.com
Surface tension concept vector illustration 21669879 Vector Art at Vecteezy Surface Tension Summary On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. Surface tension is a physical property defined as the amount of force required per unit area to expand. Surface Tension Summary.
From byjus.com
What is surface tension? Surface Tension Summary A fluid surface has a tendency to occupy as little. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Learn what is surface tension, the. Surface Tension Summary.
From www.engineeringinhindi.com
Surface tension in Hindi Surface Tension Summary A fluid surface has a tendency to occupy as little. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Surface tension is the attractive force. Surface Tension Summary.
From www.toppr.com
The unit of surface tension may be expressed as Surface Tension Summary Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Quantifies surface tension as the tension force exerted per unit length, represented as: This phenomenon can be observed. Surface Tension Summary.
From scienceinfo.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences Surface Tension Summary Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Quantifies surface tension as the tension force exerted per unit length, represented as: Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. A fluid surface has a. Surface Tension Summary.
From proper-cooking.info
Surface Tension Examples In Nature Surface Tension Summary Quantifies surface tension as the tension force exerted per unit length, represented as: Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to calculate it using the formula f/l. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface.. Surface Tension Summary.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid Surface Tension Summary Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. This phenomenon can be observed in the nearly. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to calculate it using the formula f/l. Surface tension, property. Surface Tension Summary.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Surface Tension Summary This phenomenon can be observed in the nearly. Quantifies surface tension as the tension force exerted per unit length, represented as: Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface. Surface Tension Summary.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments Surface Tension Summary On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. A fluid surface has a tendency to occupy as little. Quantifies surface tension as the tension force exerted per unit length, represented as: Surface tension is a physical property defined as the amount of force required per unit area. Surface Tension Summary.
From www.studocu.com
Measurement of surface tension by capillary tube method ment of Surface Tension Summary This phenomenon can be observed in the nearly. Quantifies surface tension as the tension force exerted per unit length, represented as: A fluid surface has a tendency to occupy as little. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to calculate it using the formula f/l. Surface tension, property. Surface Tension Summary.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension Summary This phenomenon can be observed in the nearly. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to calculate it using the formula f/l. Quantifies surface tension as the. Surface Tension Summary.
From vacationploaty.blogspot.com
Unit Of Surface Tension vacationploaty Surface Tension Summary Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to. Surface Tension Summary.
From testbook.com
Surface Tension & Capillarity with Application, Formula & Examples Surface Tension Summary A fluid surface has a tendency to occupy as little. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Surface tension, property of a liquid surface displayed. Surface Tension Summary.
From brainly.in
1. Define surface tension. Write formula, unit and dimension of surface Surface Tension Summary Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This phenomenon can be observed in the nearly. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. Learn what is surface tension, the tendency of fluid surfaces to shrink. Surface Tension Summary.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Surface Tension Summary A fluid surface has a tendency to occupy as little. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Surface tension is a physical property defined as the amount. Surface Tension Summary.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Surface Tension Summary Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. A fluid surface has a tendency to occupy as little. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. This phenomenon can be observed in. Surface Tension Summary.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Surface Tension Summary This phenomenon can be observed in the nearly. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. On account of the cohesive forces between the. Surface Tension Summary.
From www.linkedin.com
What is Surface Tension? Surface Tension Summary Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. This phenomenon can be observed in the nearly. Quantifies surface tension as the tension force exerted per unit length, represented as: Surface tension, property of a liquid surface displayed by its acting as if it were a. Surface Tension Summary.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Surface Tension Summary Quantifies surface tension as the tension force exerted per unit length, represented as: Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to calculate it using the formula f/l. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. On account. Surface Tension Summary.
From www.learnatnoon.com
What is Surface Tension in Physics? Surface Tension Summary Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. Quantifies surface tension as the tension force exerted per unit length, represented as: Surface tension is a physical property defined. Surface Tension Summary.
From www.studypool.com
SOLUTION Surface tension theory Studypool Surface Tension Summary Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid,. Surface Tension Summary.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Surface Tension Summary A fluid surface has a tendency to occupy as little. This phenomenon can be observed in the nearly. On account of the cohesive forces between the molecules of a liquid the free surface of a liquid always behaves like. Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to calculate. Surface Tension Summary.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Surface Tension Summary Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This phenomenon can be observed in the nearly. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. On account of the cohesive forces between the molecules of a liquid. Surface Tension Summary.
From www.vrogue.co
Surface Tension And Viscosity Are Used To Describe vrogue.co Surface Tension Summary Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. On account of the cohesive forces between the molecules of a liquid the free surface of a liquid. Surface Tension Summary.
From www.studocu.com
Surface Tension Problems Subject Physics Surface Tension Problems Surface Tension Summary A fluid surface has a tendency to occupy as little. This phenomenon can be observed in the nearly. Quantifies surface tension as the tension force exerted per unit length, represented as: Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface. Surface tension is a physical property defined as. Surface Tension Summary.
From www.studypool.com
SOLUTION Surface tension of milk Studypool Surface Tension Summary Learn what is surface tension, the tendency of fluid surfaces to shrink into the minimum area possible, and how to calculate it using the formula f/l. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. On account of the cohesive forces between the molecules of a. Surface Tension Summary.