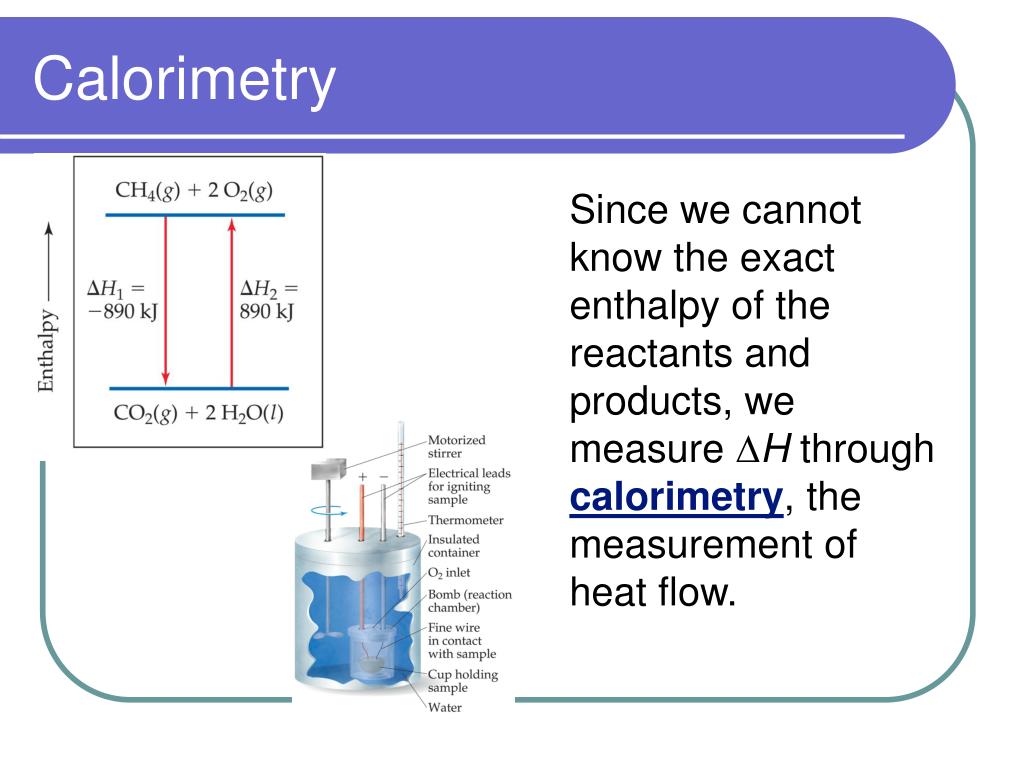
Calorimetry Lab Thermodynamics . In either case, we can measure q by measuring a change in t. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. In the laboratory, heat flow is measured in an apparatus called a calorimeter. Reactions are usually done at either constant v (in a closed container) or constant p (open to the atmosphere). A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Compare heat flow from hot to cold objects in an ideal. A calorimeter is a device used to determine heat.
from www.slideserve.com
Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. In the laboratory, heat flow is measured in an apparatus called a calorimeter. To do so, the heat is exchanged with a calibrated object (calorimeter). Reactions are usually done at either constant v (in a closed container) or constant p (open to the atmosphere). In either case, we can measure q by measuring a change in t. A calorimeter is a device used to determine heat.
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation
Calorimetry Lab Thermodynamics Compare heat flow from hot to cold objects in an ideal. Compare heat flow from hot to cold objects in an ideal. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Apply the first law of thermodynamics to calorimetry. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. In either case, we can measure q by measuring a change in t. In the laboratory, heat flow is measured in an apparatus called a calorimeter. To do so, the heat is exchanged with a calibrated object. A calorimeter is a device used to determine heat. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. Reactions are usually done at either constant v (in a closed container) or constant p (open to the atmosphere).
From www.labster.com
Calorimetry Using a bomb calorimeter Virtual Lab Calorimetry Lab Thermodynamics Calorimetry is used to measure amounts of heat transferred to or from a substance. In either case, we can measure q by measuring a change in t. To do so, the heat is exchanged with a calibrated object. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. To do so, the heat. Calorimetry Lab Thermodynamics.
From www.youtube.com
Thermodynamics Calorimetry Part I YouTube Calorimetry Lab Thermodynamics To do so, the heat is exchanged with a calibrated object (calorimeter). Compare heat flow from hot to cold objects in an ideal. A calorimeter is a device used to determine heat. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure amounts of heat transferred to or. Calorimetry Lab Thermodynamics.
From kaffee.50webs.com
Lab Calorimetry Calorimetry Lab Thermodynamics Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. Apply the first law of thermodynamics to calorimetry. To do so, the heat is exchanged with a calibrated object. Reactions are usually done at either constant v (in a closed container) or. Calorimetry Lab Thermodynamics.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Calorimetry Lab Thermodynamics A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. In either case, we can measure q by measuring a change in t.. Calorimetry Lab Thermodynamics.
From www.chegg.com
Bomb Calorimeter Lab, for Thermodynamics. Please Calorimetry Lab Thermodynamics Calorimetry is used to measure amounts of heat transferred to or from a substance. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. In either case, we can measure q by measuring a change in t. Reactions are usually done at either constant v (in a closed. Calorimetry Lab Thermodynamics.
From www.youtube.com
Thermochemistry Enthalpy and Coffee Cup Calorimeter. YouTube Calorimetry Lab Thermodynamics In the laboratory, heat flow is measured in an apparatus called a calorimeter. Compare heat flow from hot to cold objects in an ideal. A calorimeter is a device used to determine heat. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry. Calorimetry Lab Thermodynamics.
From www.youtube.com
Introduction to Chemical and Biochemical Thermodynamic Calorimetry Calorimetry Lab Thermodynamics Reactions are usually done at either constant v (in a closed container) or constant p (open to the atmosphere). A calorimeter is a device used to determine heat. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Apply the. Calorimetry Lab Thermodynamics.
From users.highland.edu
Calorimetry Calorimetry Lab Thermodynamics In either case, we can measure q by measuring a change in t. Reactions are usually done at either constant v (in a closed container) or constant p (open to the atmosphere). A calorimeter is a device used to determine heat. Apply the first law of thermodynamics to calorimetry. To do so, the heat is exchanged with a calibrated object.. Calorimetry Lab Thermodynamics.
From www.youtube.com
Calorimetry Part I Lab Version) YouTube Calorimetry Lab Thermodynamics Compare heat flow from hot to cold objects in an ideal. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Reactions are usually done at either constant v (in a closed container) or constant p (open to the atmosphere). In either case, we can. Calorimetry Lab Thermodynamics.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation Calorimetry Lab Thermodynamics In either case, we can measure q by measuring a change in t. Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal. Reactions are usually done at either constant v (in a closed container) or constant p (open to the atmosphere). Calorimetry is used. Calorimetry Lab Thermodynamics.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Lab Thermodynamics To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to determine heat. In either case, we can measure q by measuring a change in t. Calorimetry is used to measure amounts of heat transferred to or from a. Calorimetry Lab Thermodynamics.
From 2012books.lardbucket.org
Calorimetry Calorimetry Lab Thermodynamics Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Reactions are usually done at either constant v (in a closed container) or constant p (open to the atmosphere). A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter. Calorimetry Lab Thermodynamics.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Lab Thermodynamics In the laboratory, heat flow is measured in an apparatus called a calorimeter. To do so, the heat is exchanged with a calibrated object (calorimeter). Reactions are usually done at either constant v (in a closed container) or constant p (open to the atmosphere). Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is. Calorimetry Lab Thermodynamics.
From www.mheducation.com
What is McGraw Hill Virtual Labs? McGraw Hill Higher Education Calorimetry Lab Thermodynamics Apply the first law of thermodynamics to calorimetry. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or. Calorimetry Lab Thermodynamics.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Calorimetry Lab Thermodynamics Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Compare heat flow from hot to cold objects in an ideal. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. A container that prevents. Calorimetry Lab Thermodynamics.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Lab Thermodynamics Compare heat flow from hot to cold objects in an ideal. Calorimetry is used to measure amounts of heat transferred to or from a substance. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. In either case, we can. Calorimetry Lab Thermodynamics.
From www.scribd.com
Lab 4 Calorimetry Lab PDF Temperature Heat Capacity Calorimetry Lab Thermodynamics Compare heat flow from hot to cold objects in an ideal. In either case, we can measure q by measuring a change in t. To do so, the heat is exchanged with a calibrated object. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Reactions are usually done at either constant v. Calorimetry Lab Thermodynamics.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Calorimetry Lab Thermodynamics Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to determine heat. To do so, the heat is exchanged with a calibrated object (calorimeter). Reactions are usually done at either constant v (in a closed container) or constant p (open to. Calorimetry Lab Thermodynamics.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimetry Lab Thermodynamics Reactions are usually done at either constant v (in a closed container) or constant p (open to the atmosphere). A calorimeter is a device used to determine heat. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. A. Calorimetry Lab Thermodynamics.
From www.studocu.com
CHEM155 WI23 Lab ManualExpt 1 Calorimetry EXPERIMENT 1 Calorimetry Lab Thermodynamics Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). In either case, we can measure q by measuring a change in t. In the laboratory, heat flow is measured in an apparatus called a calorimeter. A calorimeter is a device used to determine. Calorimetry Lab Thermodynamics.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Calorimetry Lab Thermodynamics Calorimetry is used to measure amounts of heat transferred to or from a substance. In the laboratory, heat flow is measured in an apparatus called a calorimeter. To do so, the heat is exchanged with a calibrated object. In either case, we can measure q by measuring a change in t. A container that prevents heat transfer in or out. Calorimetry Lab Thermodynamics.
From chemistrytalk.org
Calorimetry ChemTalk Calorimetry Lab Thermodynamics In the laboratory, heat flow is measured in an apparatus called a calorimeter. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. In either case, we can measure q by measuring a change. Calorimetry Lab Thermodynamics.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter Calorimetry Lab Thermodynamics To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to determine heat. Compare heat flow from hot to cold objects in an ideal. Calorimetry is used to measure amounts of heat transferred to or. Calorimetry Lab Thermodynamics.
From www.pinterest.com
Calorimetry Lab Report in 2022 Science diagrams, Lab report Calorimetry Lab Thermodynamics A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry is used to measure amounts of heat transferred to or from a substance. In the laboratory, heat flow is measured in an apparatus called a calorimeter. Calorimetry is the. Calorimetry Lab Thermodynamics.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimetry Lab Thermodynamics A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. In either case, we can measure q by measuring a change in t. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is the. Calorimetry Lab Thermodynamics.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Lab Thermodynamics A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Reactions are usually done at either constant v (in a closed container) or constant p (open to the atmosphere). In the laboratory, heat flow is measured in an apparatus called. Calorimetry Lab Thermodynamics.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Lab Thermodynamics Reactions are usually done at either constant v (in a closed container) or constant p (open to the atmosphere). To do so, the heat is exchanged with a calibrated object (calorimeter). Compare heat flow from hot to cold objects in an ideal. A calorimeter is a device used to determine heat. In the laboratory, heat flow is measured in an. Calorimetry Lab Thermodynamics.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Lab Thermodynamics In the laboratory, heat flow is measured in an apparatus called a calorimeter. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred. Calorimetry Lab Thermodynamics.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Calorimetry Lab Thermodynamics A calorimeter is a device used to determine heat. To do so, the heat is exchanged with a calibrated object (calorimeter). In either case, we can measure q by measuring a change in t. Calorimetry is used to measure amounts of heat transferred to or from a substance. Reactions are usually done at either constant v (in a closed container). Calorimetry Lab Thermodynamics.
From www.scribd.com
GC2 Thermodynamics and Calorimetry PDF Heat Calorimetry Calorimetry Lab Thermodynamics A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure. Calorimetry Lab Thermodynamics.
From studylib.net
experiment 9 Thermochemistry Calorimetry Calorimetry Lab Thermodynamics Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. To do so, the heat is exchanged with a calibrated object. Compare heat flow from hot to cold objects in an ideal. A calorimeter is a device used to determine heat. To do so, the heat is exchanged with a calibrated object (calorimeter).. Calorimetry Lab Thermodynamics.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Lab Thermodynamics Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Reactions are usually done at either constant v (in a closed container) or. Calorimetry Lab Thermodynamics.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Lab Thermodynamics Reactions are usually done at either constant v (in a closed container) or constant p (open to the atmosphere). Compare heat flow from hot to cold objects in an ideal. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to determine heat. In the laboratory, heat. Calorimetry Lab Thermodynamics.
From www.learner.org
The Energy in Chemical Reactions Thermodynamics and Enthalpy Calorimetry Lab Thermodynamics In either case, we can measure q by measuring a change in t. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. To do so, the heat is exchanged with a calibrated object (calorimeter). A container that prevents heat transfer in or out is called a calorimeter, and the use of a. Calorimetry Lab Thermodynamics.
From www.scribd.com
Calorimetry Lab Data Sheet PDF Branches Of Thermodynamics Heat Calorimetry Lab Thermodynamics Apply the first law of thermodynamics to calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to determine heat. A container that prevents heat transfer in or out is called a calorimeter, and. Calorimetry Lab Thermodynamics.