Is Mg/A Concentration . The most common units are molarity, molality, normality, mass percent, volume percent, and mole. Use the terms concentrated and dilute to describe the relative concentration of a solution. How to calculate units of concentration. A concentration on an m/v basis is the. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. In chemistry, a solution's concentration is how much of a dissolvable. There are a number of ways to express the relative amounts of solute and solvent in a solution. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; Which unit you use depends on how you intend to use the chemical solution.
from www.nagwa.com
Which unit you use depends on how you intend to use the chemical solution. The most common units are molarity, molality, normality, mass percent, volume percent, and mole. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. In chemistry, a solution's concentration is how much of a dissolvable. There are a number of ways to express the relative amounts of solute and solvent in a solution. How to calculate units of concentration. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; Use the terms concentrated and dilute to describe the relative concentration of a solution. A concentration on an m/v basis is the.
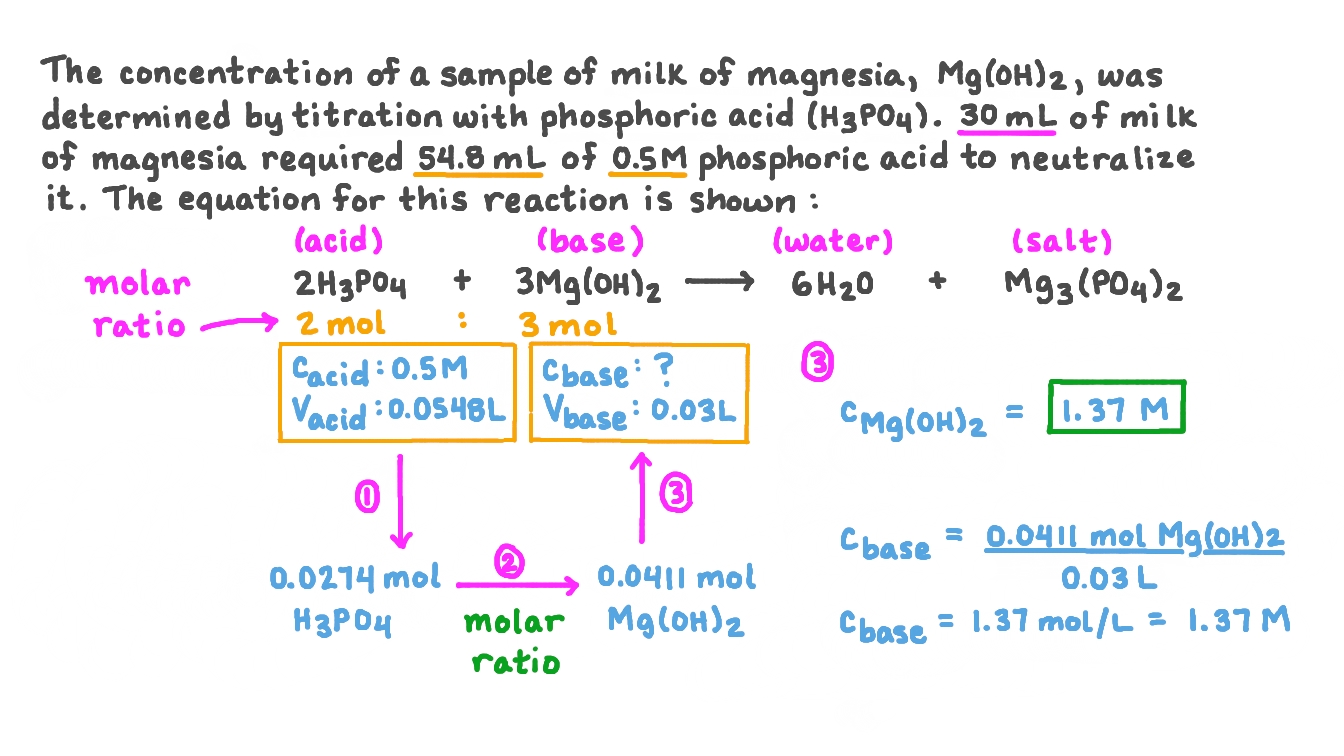
Question Video Calculating the Molar Concentration of Mg(OH)₂ Using
Is Mg/A Concentration In chemistry, a solution's concentration is how much of a dissolvable. The most common units are molarity, molality, normality, mass percent, volume percent, and mole. There are a number of ways to express the relative amounts of solute and solvent in a solution. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. A concentration on an m/v basis is the. In chemistry, a solution's concentration is how much of a dissolvable. Use the terms concentrated and dilute to describe the relative concentration of a solution. Which unit you use depends on how you intend to use the chemical solution. How to calculate units of concentration.
From www.chegg.com
Solved The plasma concentrations measured after intravenous Is Mg/A Concentration In chemistry, a solution's concentration is how much of a dissolvable. There are a number of ways to express the relative amounts of solute and solvent in a solution. A concentration on an m/v basis is the. How to calculate units of concentration. A concentration expressed on an m/m basis is equal to the number of grams of solute per. Is Mg/A Concentration.
From www.transtutors.com
(Solved) Calculate the 0.45 ppm NO2 concentration at 25oC and 1 atm Is Mg/A Concentration Which unit you use depends on how you intend to use the chemical solution. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. There are a number of ways to express the relative amounts of solute and solvent in a solution. In chemistry, a solution's concentration is how much of a. Is Mg/A Concentration.
From www.chegg.com
Solved Concentration (mg/L). Absorbance Solution 1 Solution Is Mg/A Concentration How to calculate units of concentration. Use the terms concentrated and dilute to describe the relative concentration of a solution. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; In chemistry, a solution's concentration is how much of a dissolvable. Which unit you use depends on how you intend. Is Mg/A Concentration.
From www.slideserve.com
PPT Concentration & Solutions Part 2 PowerPoint Presentation ID Is Mg/A Concentration Which unit you use depends on how you intend to use the chemical solution. There are a number of ways to express the relative amounts of solute and solvent in a solution. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. A concentration expressed on an m/m basis is equal to. Is Mg/A Concentration.
From www.nagwa.com
Question Video Calculating the Molar Concentration of Mg(OH)₂ Using Is Mg/A Concentration There are a number of ways to express the relative amounts of solute and solvent in a solution. The most common units are molarity, molality, normality, mass percent, volume percent, and mole. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; Use the terms concentrated and dilute to describe. Is Mg/A Concentration.
From www.pinterest.fr
Units of Concentration Should've probably printed this for Is Mg/A Concentration The most common units are molarity, molality, normality, mass percent, volume percent, and mole. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; In chemistry, a solution's concentration is how much of a dissolvable. Once you have identified the solute and solvent in a solution, you are ready to. Is Mg/A Concentration.
From www.coursehero.com
[Solved] the Iv with nitroglycerin is infusing at 6 ml/hr. the Is Mg/A Concentration In chemistry, a solution's concentration is how much of a dissolvable. How to calculate units of concentration. The most common units are molarity, molality, normality, mass percent, volume percent, and mole. Which unit you use depends on how you intend to use the chemical solution. A concentration expressed on an m/m basis is equal to the number of grams of. Is Mg/A Concentration.
From www.coursehero.com
[Solved] 1. Calculate the drug concentration 8 hours after a continuous Is Mg/A Concentration A concentration on an m/v basis is the. Which unit you use depends on how you intend to use the chemical solution. The most common units are molarity, molality, normality, mass percent, volume percent, and mole. Use the terms concentrated and dilute to describe the relative concentration of a solution. In chemistry, a solution's concentration is how much of a. Is Mg/A Concentration.
From www.numerade.com
Required information The given table lists values for dissolved oxygen Is Mg/A Concentration In chemistry, a solution's concentration is how much of a dissolvable. Use the terms concentrated and dilute to describe the relative concentration of a solution. A concentration on an m/v basis is the. The most common units are molarity, molality, normality, mass percent, volume percent, and mole. There are a number of ways to express the relative amounts of solute. Is Mg/A Concentration.
From www.slideserve.com
PPT Concentration & Solutions Part 2 PowerPoint Presentation ID Is Mg/A Concentration How to calculate units of concentration. Which unit you use depends on how you intend to use the chemical solution. There are a number of ways to express the relative amounts of solute and solvent in a solution. In chemistry, a solution's concentration is how much of a dissolvable. A concentration expressed on an m/m basis is equal to the. Is Mg/A Concentration.
From www.researchgate.net
How to convert arsenic concentration ug/L (in solution) to mg/kg (in Is Mg/A Concentration How to calculate units of concentration. Use the terms concentrated and dilute to describe the relative concentration of a solution. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. Which unit you use depends on how you intend to use the chemical solution. A concentration expressed on an m/m basis is. Is Mg/A Concentration.
From www.drawittoknowit.com
Pharmacology Glossary Loading Dose Draw It to Know It Is Mg/A Concentration Use the terms concentrated and dilute to describe the relative concentration of a solution. The most common units are molarity, molality, normality, mass percent, volume percent, and mole. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; In chemistry, a solution's concentration is how much of a dissolvable. A. Is Mg/A Concentration.
From www.coursehero.com
[Solved] What is the concentration of a reactant after 22.0 s if the Is Mg/A Concentration The most common units are molarity, molality, normality, mass percent, volume percent, and mole. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. In chemistry, a solution's concentration is how much of a dissolvable. There are a number of ways to express the relative amounts of solute and solvent in a. Is Mg/A Concentration.
From www.slideserve.com
PPT Solutions Part II Units of Concentration (loosely from Jespersen Is Mg/A Concentration The most common units are molarity, molality, normality, mass percent, volume percent, and mole. Use the terms concentrated and dilute to describe the relative concentration of a solution. In chemistry, a solution's concentration is how much of a dissolvable. Which unit you use depends on how you intend to use the chemical solution. How to calculate units of concentration. Once. Is Mg/A Concentration.
From revisechemistry.uk
Quantitative Chemistry AQA C3 revisechemistry.uk Is Mg/A Concentration In chemistry, a solution's concentration is how much of a dissolvable. The most common units are molarity, molality, normality, mass percent, volume percent, and mole. Use the terms concentrated and dilute to describe the relative concentration of a solution. Which unit you use depends on how you intend to use the chemical solution. Once you have identified the solute and. Is Mg/A Concentration.
From www.chegg.com
Solved The concentration, C, of a drug (in mg/L) in the Is Mg/A Concentration The most common units are molarity, molality, normality, mass percent, volume percent, and mole. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. A concentration on an m/v basis is the.. Is Mg/A Concentration.
From www.numerade.com
SOLVED 34. Calculate the Filtration Fraction. P = Plasma Concentration Is Mg/A Concentration Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. A concentration on an m/v basis is the. Which unit you use depends on how you intend to use the chemical solution. How to calculate units of concentration. The most common units are molarity, molality, normality, mass percent, volume percent, and mole.. Is Mg/A Concentration.
From www.coursehero.com
[Solved] A sample of ground water has 150 mg/L of Ca2+ and 60 mg/L of Is Mg/A Concentration How to calculate units of concentration. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; There are a number of ways to express the relative amounts of solute and solvent in. Is Mg/A Concentration.
From www.numerade.com
SOLVEDIf the concentration of Mg^2+ ion in seawater is 1350 mg / L Is Mg/A Concentration Use the terms concentrated and dilute to describe the relative concentration of a solution. A concentration on an m/v basis is the. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution;. Is Mg/A Concentration.
From www.coursehero.com
[Solved] Medication with strength 125 mg/5 mL has been ordered at 20 mg Is Mg/A Concentration A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; The most common units are molarity, molality, normality, mass percent, volume percent, and mole. There are a number of ways to express the relative amounts of solute and solvent in a solution. Use the terms concentrated and dilute to describe. Is Mg/A Concentration.
From www.coursehero.com
[Solved] 1. Calculate the drug concentration 8 hours after a continuous Is Mg/A Concentration Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. A concentration on an m/v basis is the. There are a number of ways to express the relative amounts of solute and solvent in a solution. The most common units are molarity, molality, normality, mass percent, volume percent, and mole. A concentration. Is Mg/A Concentration.
From www.slideserve.com
PPT ATMOSPHERIC CONCENTRATION UNITS PowerPoint Presentation, free Is Mg/A Concentration Which unit you use depends on how you intend to use the chemical solution. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; There are a number of ways to express. Is Mg/A Concentration.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2ad Concentration vs Is Mg/A Concentration There are a number of ways to express the relative amounts of solute and solvent in a solution. How to calculate units of concentration. Use the terms concentrated and dilute to describe the relative concentration of a solution. Which unit you use depends on how you intend to use the chemical solution. A concentration expressed on an m/m basis is. Is Mg/A Concentration.
From www.toppr.com
Concentration of glucose (C,H,0.) in normal blood is approximately 90 Is Mg/A Concentration There are a number of ways to express the relative amounts of solute and solvent in a solution. In chemistry, a solution's concentration is how much of a dissolvable. A concentration on an m/v basis is the. The most common units are molarity, molality, normality, mass percent, volume percent, and mole. How to calculate units of concentration. Use the terms. Is Mg/A Concentration.
From www.chegg.com
Solved 1. Calculate the final concentration of a solution Is Mg/A Concentration A concentration on an m/v basis is the. The most common units are molarity, molality, normality, mass percent, volume percent, and mole. Which unit you use depends on how you intend to use the chemical solution. How to calculate units of concentration. Use the terms concentrated and dilute to describe the relative concentration of a solution. In chemistry, a solution's. Is Mg/A Concentration.
From www.chegg.com
Solved The concentration C in milligrams per milliliter Is Mg/A Concentration In chemistry, a solution's concentration is how much of a dissolvable. A concentration on an m/v basis is the. Use the terms concentrated and dilute to describe the relative concentration of a solution. Which unit you use depends on how you intend to use the chemical solution. There are a number of ways to express the relative amounts of solute. Is Mg/A Concentration.
From www.youtube.com
Concentration to "mg/ml" or "mg/g". A simple conversion technique for Is Mg/A Concentration In chemistry, a solution's concentration is how much of a dissolvable. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; Which unit you use depends on how you intend to use the chemical solution. There are a number of ways to express the relative amounts of solute and solvent. Is Mg/A Concentration.
From www.chegg.com
Solved A patient was given a single dose of 30 mg of a drug Is Mg/A Concentration Which unit you use depends on how you intend to use the chemical solution. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; A concentration on an m/v basis is the. How to calculate units of concentration. The most common units are molarity, molality, normality, mass percent, volume percent,. Is Mg/A Concentration.
From www.chegg.com
Solved The Concentration Of OH In A Saturated Solution I... Is Mg/A Concentration The most common units are molarity, molality, normality, mass percent, volume percent, and mole. In chemistry, a solution's concentration is how much of a dissolvable. A concentration on an m/v basis is the. Use the terms concentrated and dilute to describe the relative concentration of a solution. How to calculate units of concentration. There are a number of ways to. Is Mg/A Concentration.
From www.chegg.com
Solved 7. A single 500 mg IV bolus injection of cetamandole Is Mg/A Concentration There are a number of ways to express the relative amounts of solute and solvent in a solution. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. A concentration on an m/v basis is the. In chemistry, a solution's concentration is how much of a dissolvable. A concentration expressed on an. Is Mg/A Concentration.
From www.chegg.com
Solved The concentration of a drug in a patient's Is Mg/A Concentration How to calculate units of concentration. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution; In chemistry, a solution's concentration is how much of a dissolvable. There are a number of ways to express the relative amounts of solute and solvent in a solution. A concentration on an m/v. Is Mg/A Concentration.
From www.chegg.com
Solved Determine the saturation concentration, in mg/L, of Is Mg/A Concentration Which unit you use depends on how you intend to use the chemical solution. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. Use the terms concentrated and dilute to describe the relative concentration of a solution. There are a number of ways to express the relative amounts of solute and. Is Mg/A Concentration.
From www.youtube.com
Calculate Concentration (Example) YouTube Is Mg/A Concentration The most common units are molarity, molality, normality, mass percent, volume percent, and mole. There are a number of ways to express the relative amounts of solute and solvent in a solution. How to calculate units of concentration. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. In chemistry, a solution's. Is Mg/A Concentration.
From www.researchgate.net
How do I get (mg/g) concentration value from (mg/l) units? ResearchGate Is Mg/A Concentration A concentration on an m/v basis is the. Which unit you use depends on how you intend to use the chemical solution. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. A concentration expressed on an m/m basis is equal to the number of grams of solute per gram of solution;. Is Mg/A Concentration.
From www.chegg.com
Glucose Concentration (mg/dL) Data Table 1 Enzyme Is Mg/A Concentration Use the terms concentrated and dilute to describe the relative concentration of a solution. A concentration on an m/v basis is the. In chemistry, a solution's concentration is how much of a dissolvable. Once you have identified the solute and solvent in a solution, you are ready to determine its concentration. There are a number of ways to express the. Is Mg/A Concentration.