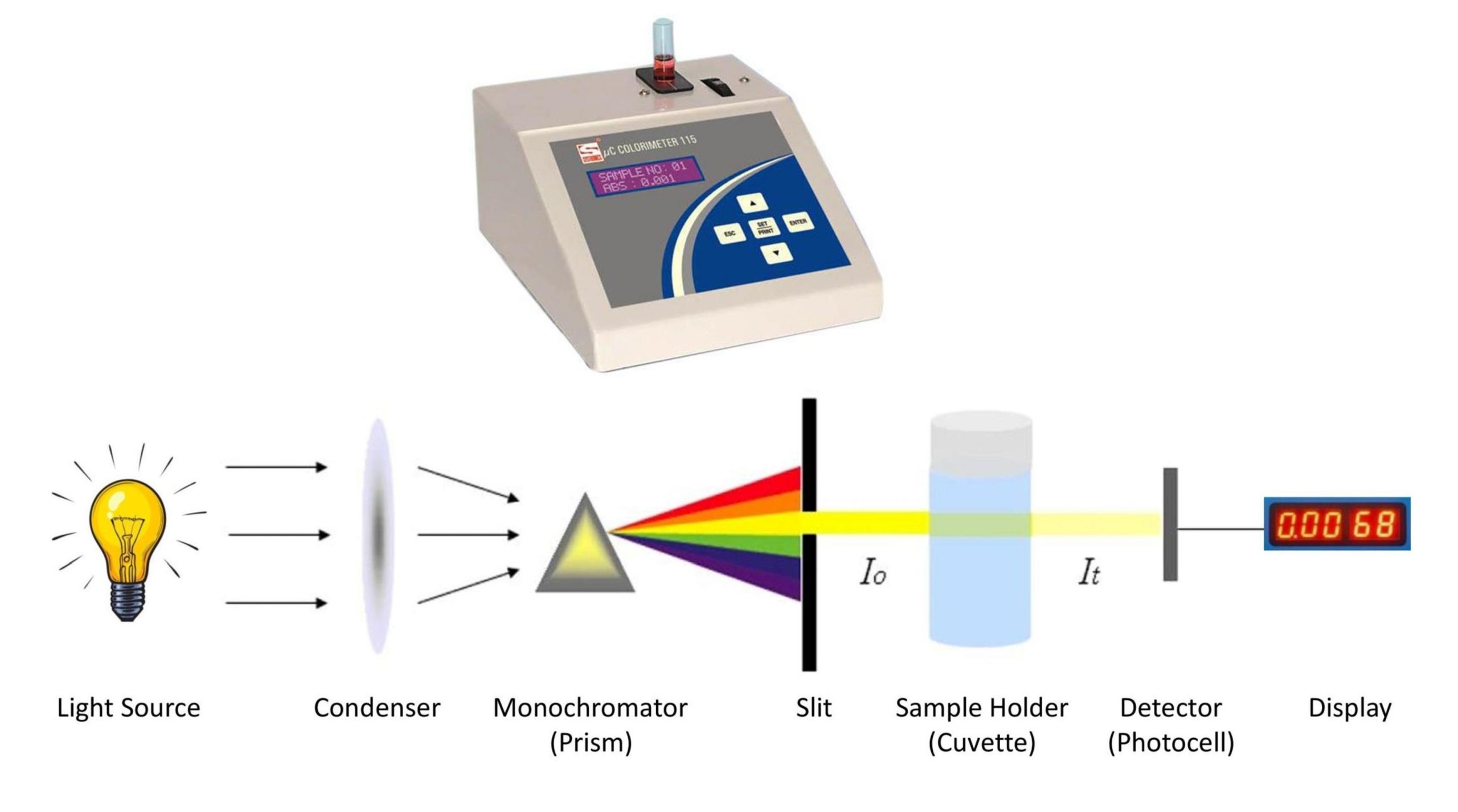
Beer Lambert Law In Colorimetry . The colorimeter was invented in the year 1870 by louis j duboscq. A = abc, where a is the absorbance of the solution, a is the molar. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship is known as beer’s law and is given by the equation: It refers to a device which helps specific solutions to absorb a particular wavelength of light. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of.
from laboratorytests.org
This relationship is known as beer’s law and is given by the equation: A = abc, where a is the absorbance of the solution, a is the molar. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. It refers to a device which helps specific solutions to absorb a particular wavelength of light. The colorimeter was invented in the year 1870 by louis j duboscq. When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a.
Colorimeter Principle, Instrumentation and Uses
Beer Lambert Law In Colorimetry Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship is known as beer’s law and is given by the equation: When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. A = abc, where a is the absorbance of the solution, a is the molar. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. It refers to a device which helps specific solutions to absorb a particular wavelength of light. The colorimeter was invented in the year 1870 by louis j duboscq. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.
From www.youtube.com
Introduction to Colorimetry Verify LambertBeer Law Experimental Beer Lambert Law In Colorimetry Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. The colorimeter was invented in the year 1870 by louis j duboscq. This relationship is known. Beer Lambert Law In Colorimetry.
From ceyopxll.blob.core.windows.net
Beer Lambert Law Notes at Christina Wadsworth blog Beer Lambert Law In Colorimetry The colorimeter was invented in the year 1870 by louis j duboscq. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. It refers to a device which helps specific solutions to absorb a particular wavelength of light. “the thicker the glass, the darker the. Beer Lambert Law In Colorimetry.
From www.youtube.com
Beer's Law Overview YouTube Beer Lambert Law In Colorimetry The colorimeter was invented in the year 1870 by louis j duboscq. When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. It refers to a device which helps specific solutions to absorb a particular wavelength of light. “the thicker the glass, the darker the brew, the less the light that. Beer Lambert Law In Colorimetry.
From www.youtube.com
LambertBeer's Law Visible Spectroscopy Colorimetry By Prof. K Beer Lambert Law In Colorimetry When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. This relationship is known as beer’s law and is given by the equation: A = abc, where a is the absorbance of the solution, a is the molar. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law In Colorimetry.
From www.youtube.com
Beer Lambert Law Demonstration Tamil Colorimeter Beer Lambert Law In Colorimetry This relationship is known as beer’s law and is given by the equation: “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with. Beer Lambert Law In Colorimetry.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer Lambert Law In Colorimetry When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship is known as beer’s law and is given by the equation: A = abc,. Beer Lambert Law In Colorimetry.
From laboratorytests.org
Colorimeter Principle, Instrumentation and Uses Beer Lambert Law In Colorimetry The colorimeter was invented in the year 1870 by louis j duboscq. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore. Beer Lambert Law In Colorimetry.
From www.youtube.com
Lambert Beer's Law Colorimetry Dr. G L Jadav YouTube Beer Lambert Law In Colorimetry Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship is known as beer’s law and is given by the equation: A = abc, where a is the absorbance of the solution, a is the molar. The colorimeter was invented in the year. Beer Lambert Law In Colorimetry.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer Lambert Law In Colorimetry The colorimeter was invented in the year 1870 by louis j duboscq. This relationship is known as beer’s law and is given by the equation: It refers to a device which helps specific solutions to absorb a particular wavelength of light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the. Beer Lambert Law In Colorimetry.
From joibnsohl.blob.core.windows.net
Spectrophotometry Learn The BeerLambert Law With Absorbance Beer Lambert Law In Colorimetry Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. A = abc,. Beer Lambert Law In Colorimetry.
From www.slideserve.com
PPT Chemical the rates of reactions PowerPoint Presentation Beer Lambert Law In Colorimetry The colorimeter was invented in the year 1870 by louis j duboscq. When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. “the thicker the glass,. Beer Lambert Law In Colorimetry.
From www.slideshare.net
beer lamberts law, colorimetery, nephlometry and turbidimetry Beer Lambert Law In Colorimetry A = abc, where a is the absorbance of the solution, a is the molar. It refers to a device which helps specific solutions to absorb a particular wavelength of light. The colorimeter was invented in the year 1870 by louis j duboscq. When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in. Beer Lambert Law In Colorimetry.
From www.youtube.com
Colorimetry Verify LambertBeer's Law & To find Concentration of Beer Lambert Law In Colorimetry It refers to a device which helps specific solutions to absorb a particular wavelength of light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of.. Beer Lambert Law In Colorimetry.
From stuff.iorodeo.com
Lab 2 Beer’s Law and Molar Extinction Coefficients — Colorimeter User Beer Lambert Law In Colorimetry “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. The colorimeter was invented in the year 1870 by louis j duboscq. A = abc, where a is the absorbance of the solution, a is the molar. Since. Beer Lambert Law In Colorimetry.
From www.youtube.com
Colorimetryverify LambertBeer's Law YouTube Beer Lambert Law In Colorimetry When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. This relationship is known as beer’s law and is given by the equation: It refers to a device which helps specific solutions to absorb a particular wavelength of light. “the thicker the glass, the darker the brew, the less the light. Beer Lambert Law In Colorimetry.
From www.youtube.com
Application of BeerLambert's Law_Colorimetry YouTube Beer Lambert Law In Colorimetry The colorimeter was invented in the year 1870 by louis j duboscq. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. A = abc, where a is the absorbance of the solution, a is the molar. When. Beer Lambert Law In Colorimetry.
From www.slideshare.net
beer lamberts law, colorimetery, nephlometry and turbidimetry Beer Lambert Law In Colorimetry A = abc, where a is the absorbance of the solution, a is the molar. When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The. Beer Lambert Law In Colorimetry.
From www.youtube.com
BSC 2nd year 3rd semester zoology unit 7 [ colorimetry , Beer and Beer Lambert Law In Colorimetry Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. A = abc, where a is the absorbance of the solution, a is the molar. This relationship is known as beer’s law and is given by the equation: The colorimeter was invented in the year. Beer Lambert Law In Colorimetry.
From www.youtube.com
Theory of Colorimetry & Colorimeter Beer's & Lambert's Law YouTube Beer Lambert Law In Colorimetry Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. It refers to a device which helps specific solutions to absorb a particular wavelength of light. The colorimeter was invented in the year 1870 by louis j duboscq. When monochromatic light passes through a colored. Beer Lambert Law In Colorimetry.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer Lambert Law In Colorimetry “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. A = abc, where a is the absorbance of the solution, a is the molar. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer Lambert Law In Colorimetry.
From loericfgn.blob.core.windows.net
Beer's Law Values at Alfred Nee blog Beer Lambert Law In Colorimetry When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. A = abc, where a is the absorbance of the solution, a is the molar. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light. Beer Lambert Law In Colorimetry.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law In Colorimetry When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. It refers to a device which helps specific solutions. Beer Lambert Law In Colorimetry.
From www.youtube.com
Colorimetry Beer Lambert’s law quiz YouTube Beer Lambert Law In Colorimetry A = abc, where a is the absorbance of the solution, a is the molar. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The colorimeter was invented in the year 1870 by louis j duboscq. “the thicker the glass, the darker the brew,. Beer Lambert Law In Colorimetry.
From chemdictionary.org
BeerLambert Law History, Definition & Example Calculation Beer Lambert Law In Colorimetry “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. A = abc, where a is the absorbance of the solution, a is the molar. The colorimeter was invented in the year 1870 by louis j duboscq. When. Beer Lambert Law In Colorimetry.
From www.youtube.com
Working of Colorimeter and Verification of Beer'sLambert's law Beer Lambert Law In Colorimetry A = abc, where a is the absorbance of the solution, a is the molar. This relationship is known as beer’s law and is given by the equation: When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. The colorimeter was invented in the year 1870 by louis j duboscq. Since. Beer Lambert Law In Colorimetry.
From www.youtube.com
Colorimetry Beer's law Lambert's law biochemistry YouTube Beer Lambert Law In Colorimetry This relationship is known as beer’s law and is given by the equation: “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. A = abc, where a is the absorbance of the solution, a is the molar.. Beer Lambert Law In Colorimetry.
From www.slideshare.net
beer lamberts law, colorimetery, nephlometry and turbidimetry Beer Lambert Law In Colorimetry Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. A = abc, where a is the absorbance of the solution, a is the molar. It. Beer Lambert Law In Colorimetry.
From www.researchgate.net
1 BeerLambert law; Xrays to solid matter interaction. Download Beer Lambert Law In Colorimetry It refers to a device which helps specific solutions to absorb a particular wavelength of light. A = abc, where a is the absorbance of the solution, a is the molar. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship is known. Beer Lambert Law In Colorimetry.
From www.slideshare.net
beer lamberts law, colorimetery, nephlometry and turbidimetry PPT Beer Lambert Law In Colorimetry When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. The colorimeter was invented in the year 1870 by louis j duboscq. This relationship is known as beer’s law and is given by the equation: “the thicker the glass, the darker the brew, the less the light that passes through.” make. Beer Lambert Law In Colorimetry.
From www.youtube.com
Spectroscopy Beer Lambert's Law YouTube Beer Lambert Law In Colorimetry Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. A = abc, where a is the absorbance of the solution, a is the molar. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute. Beer Lambert Law In Colorimetry.
From www.slideshare.net
beer lamberts law, colorimetery, nephlometry and turbidimetry Beer Lambert Law In Colorimetry “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. It refers to a device which helps specific solutions to absorb a particular wavelength of light. A = abc, where a is the absorbance of the solution, a. Beer Lambert Law In Colorimetry.
From www.scribd.com
01 Beer Lambert Law Colorimetry PDF Spectroscopy Absorbance Beer Lambert Law In Colorimetry It refers to a device which helps specific solutions to absorb a particular wavelength of light. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. This relationship is known as beer’s law and is given by the equation: A = abc, where a is. Beer Lambert Law In Colorimetry.
From cevhwrun.blob.core.windows.net
Beer Lambert Law Relationship Between Absorbance And Concentration at Beer Lambert Law In Colorimetry “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a. When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. A = abc, where a is the absorbance of. Beer Lambert Law In Colorimetry.
From www.youtube.com
Spectrophotometry BeerLambert Law. YouTube Beer Lambert Law In Colorimetry When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of. It refers to a device which helps specific solutions to absorb a particular wavelength of light. “the thicker the glass, the darker the brew, the less the light that passes through.” make colorful concentrated and dilute solutions and explore how much. Beer Lambert Law In Colorimetry.
From www.youtube.com
Colorimetry Verify Lambert Beer Law (Potassium dichromate solution) Beer Lambert Law In Colorimetry This relationship is known as beer’s law and is given by the equation: The colorimeter was invented in the year 1870 by louis j duboscq. It refers to a device which helps specific solutions to absorb a particular wavelength of light. When monochromatic light passes through a colored solution, amount of light transmitted decreases exponentially with increase in concentration of.. Beer Lambert Law In Colorimetry.