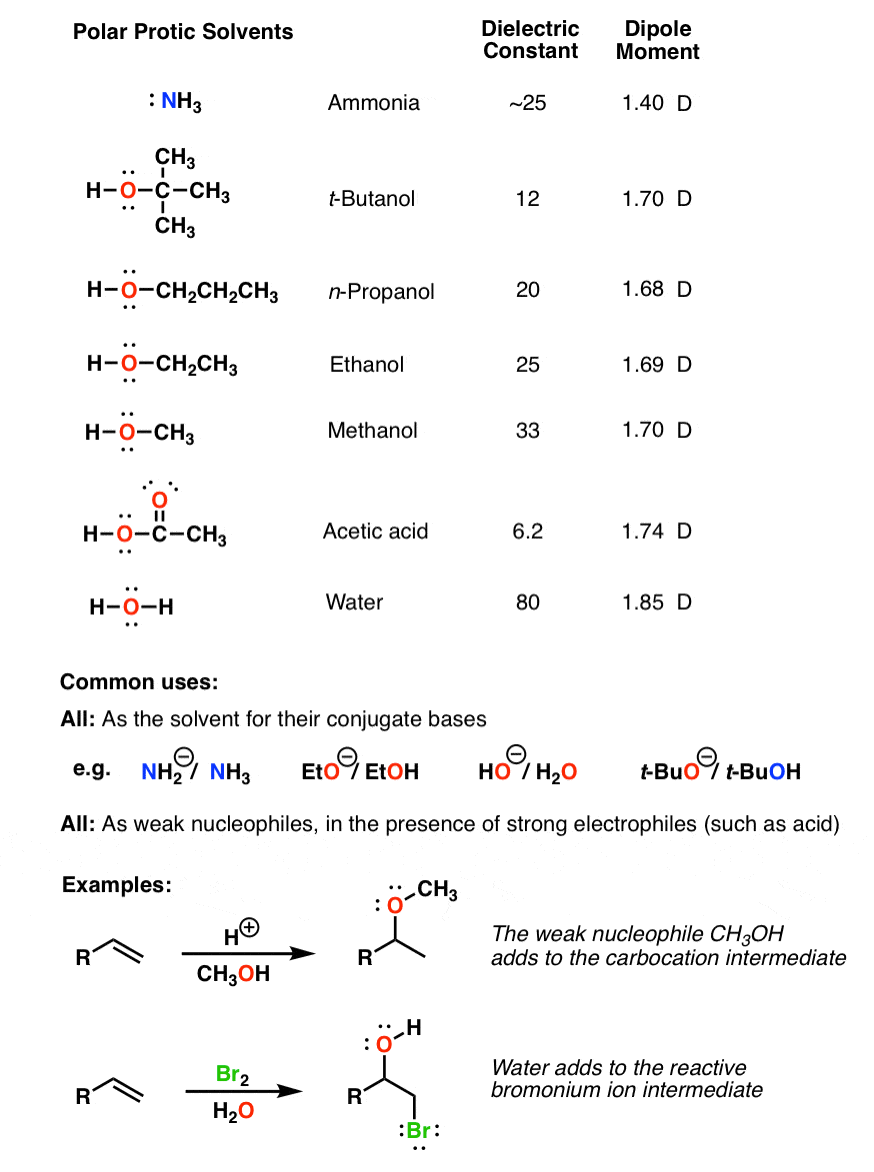
Is Acetone Polar Protic . A solvent is polar if it has a dipole moment greater than 1.6 d and a dielectric constant greater. The general guideline for solvents regarding nucleophilic substitution reaction is: Acetone is a polar aprotic solvent. Polar aprotic solvents tend to host sn2 reactions. In this example, we will use an alkyl halide (electrophile) and kf. Although their dielectric constant are. Our polar aprotic solvent will be acetone. In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; S n 1 reactions are favored by polar protic solvents (h 2 o, roh etc), and usually are solvolysis. The leaving group then breaks away. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. Both are polar but acn is a polar aprotic and metanol a polar protic.
from www.masterorganicchemistry.com
S n 1 reactions are favored by polar protic solvents (h 2 o, roh etc), and usually are solvolysis. Both are polar but acn is a polar aprotic and metanol a polar protic. In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; The leaving group then breaks away. In this example, we will use an alkyl halide (electrophile) and kf. A solvent is polar if it has a dipole moment greater than 1.6 d and a dielectric constant greater. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Acetone is a polar aprotic solvent. Our polar aprotic solvent will be acetone. Although their dielectric constant are.
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents
Is Acetone Polar Protic A solvent is polar if it has a dipole moment greater than 1.6 d and a dielectric constant greater. Acetone is a polar aprotic solvent. In this example, we will use an alkyl halide (electrophile) and kf. S n 1 reactions are favored by polar protic solvents (h 2 o, roh etc), and usually are solvolysis. In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; The general guideline for solvents regarding nucleophilic substitution reaction is: 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. Polar aprotic solvents tend to host sn2 reactions. The leaving group then breaks away. Both are polar but acn is a polar aprotic and metanol a polar protic. Although their dielectric constant are. Our polar aprotic solvent will be acetone. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. A solvent is polar if it has a dipole moment greater than 1.6 d and a dielectric constant greater.
From www.pinterest.com
Polar aprotic solvents favor SN2 while polar protic solvents favor the Is Acetone Polar Protic In this example, we will use an alkyl halide (electrophile) and kf. Polar aprotic solvents tend to host sn2 reactions. S n 1 reactions are favored by polar protic solvents (h 2 o, roh etc), and usually are solvolysis. Although their dielectric constant are. Both are polar but acn is a polar aprotic and metanol a polar protic. A solvent. Is Acetone Polar Protic.
From kandicea-ethics.blogspot.com
Polar Protic Solvents Meaning / Tryambak Srivastava On Twitter Polar Is Acetone Polar Protic In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; Both are polar but acn is a polar aprotic and metanol a polar protic. In this example, we will use an alkyl halide (electrophile) and kf. A solvent is polar if it has a dipole moment greater than 1.6 d and a dielectric constant greater.. Is Acetone Polar Protic.
From chemistrytalk.org
Polar Protic and Aprotic Solvents ChemTalk Is Acetone Polar Protic 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. The leaving group then breaks away. In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; Acetone is a polar aprotic solvent. In this example, we will use an alkyl halide (electrophile) and. Is Acetone Polar Protic.
From chemistrytalk.org
Polar Protic and Aprotic Solvents ChemTalk Is Acetone Polar Protic Both are polar but acn is a polar aprotic and metanol a polar protic. Polar aprotic solvents tend to host sn2 reactions. The general guideline for solvents regarding nucleophilic substitution reaction is: The leaving group then breaks away. Our polar aprotic solvent will be acetone. A solvent is polar if it has a dipole moment greater than 1.6 d and. Is Acetone Polar Protic.
From magdalenakruwsalazar.blogspot.com
Acetone Polar or Nonpolar Solvent MagdalenakruwSalazar Is Acetone Polar Protic Polar aprotic solvents tend to host sn2 reactions. Our polar aprotic solvent will be acetone. In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. A solvent is polar if it has. Is Acetone Polar Protic.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Is Acetone Polar Protic A solvent is polar if it has a dipole moment greater than 1.6 d and a dielectric constant greater. The leaving group then breaks away. Our polar aprotic solvent will be acetone. S n 1 reactions are favored by polar protic solvents (h 2 o, roh etc), and usually are solvolysis. Although their dielectric constant are. 23 rows however, acetone. Is Acetone Polar Protic.
From www.nagwa.com
Question Video Determining Whether Some Common Simple Molecular Is Acetone Polar Protic Our polar aprotic solvent will be acetone. S n 1 reactions are favored by polar protic solvents (h 2 o, roh etc), and usually are solvolysis. Both are polar but acn is a polar aprotic and metanol a polar protic. Acetone is a polar aprotic solvent. The leaving group then breaks away. In sn2 reactions, the nucleophile attacks the electrophile. Is Acetone Polar Protic.
From www.numerade.com
SOLVED Shown above is a representation of hydrogen bonding between two Is Acetone Polar Protic S n 1 reactions are favored by polar protic solvents (h 2 o, roh etc), and usually are solvolysis. The leaving group then breaks away. Our polar aprotic solvent will be acetone. In this example, we will use an alkyl halide (electrophile) and kf. Although their dielectric constant are. Polar aprotic solvents tend to host sn2 reactions. Acetone is a. Is Acetone Polar Protic.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 (3) The Solvent Master Organic Chemistry Is Acetone Polar Protic Polar aprotic solvents tend to host sn2 reactions. In this example, we will use an alkyl halide (electrophile) and kf. In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; Our polar aprotic solvent will be acetone. The leaving group then breaks away. S n 1 reactions are favored by polar protic solvents (h 2. Is Acetone Polar Protic.
From www.chemistrysteps.com
The Role of Solvent in SN1, SN2, E1 and E2 Reactions Chemistry Steps Is Acetone Polar Protic Polar aprotic solvents tend to host sn2 reactions. Our polar aprotic solvent will be acetone. The general guideline for solvents regarding nucleophilic substitution reaction is: 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. The leaving group then breaks away. In sn2 reactions, the nucleophile attacks. Is Acetone Polar Protic.
From www.numerade.com
SOLVED Which of the following is a polar aprotic solvent? A) Water B Is Acetone Polar Protic Both are polar but acn is a polar aprotic and metanol a polar protic. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; In this example, we will use an alkyl halide. Is Acetone Polar Protic.
From www.numerade.com
SOLVEDQuestion 34 (1 point) Example of a polar protic solvent is EtOH Is Acetone Polar Protic The leaving group then breaks away. Although their dielectric constant are. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. In this example, we. Is Acetone Polar Protic.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Master Is Acetone Polar Protic A solvent is polar if it has a dipole moment greater than 1.6 d and a dielectric constant greater. Our polar aprotic solvent will be acetone. Acetone is a polar aprotic solvent. Although their dielectric constant are. In this example, we will use an alkyl halide (electrophile) and kf. 23 rows however, acetone is still considered a polar aprotic solvent,. Is Acetone Polar Protic.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Is Acetone Polar Protic Although their dielectric constant are. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. In this example, we will use an alkyl halide (electrophile) and kf. A solvent is polar if it has a dipole moment greater than 1.6 d and a dielectric constant greater. Polar. Is Acetone Polar Protic.
From landynfinpitts.blogspot.com
Acetone Polar or Nonpolar Solvent LandynfinPitts Is Acetone Polar Protic The general guideline for solvents regarding nucleophilic substitution reaction is: Although their dielectric constant are. In this example, we will use an alkyl halide (electrophile) and kf. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. Acetone is a polar aprotic solvent. The leaving group then breaks. Is Acetone Polar Protic.
From www.slideserve.com
PPT Substitution and Elimination PowerPoint Presentation, free Is Acetone Polar Protic Our polar aprotic solvent will be acetone. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. A solvent is polar if it has a dipole moment greater than 1.6 d and a dielectric constant greater. The general guideline for solvents regarding nucleophilic substitution reaction is: Both are. Is Acetone Polar Protic.
From carlynewsturner.blogspot.com
Acetone Polar or Nonpolar Solvent Is Acetone Polar Protic 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Our polar aprotic solvent will be acetone. Polar aprotic solvents tend to host sn2 reactions. The general guideline for solvents regarding nucleophilic substitution reaction is: A solvent is polar if it has a dipole moment greater than. Is Acetone Polar Protic.
From chem-net.blogspot.com
Solvent Effects and SN2 and SN1 reactions Nucleophilic Substitution Is Acetone Polar Protic 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. The general guideline for solvents regarding nucleophilic substitution reaction is: In this example, we will use an alkyl halide (electrophile) and kf. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that. Is Acetone Polar Protic.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID289043 Is Acetone Polar Protic Polar aprotic solvents tend to host sn2 reactions. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. In this example, we will use an alkyl halide (electrophile) and kf. The general guideline for solvents regarding nucleophilic substitution reaction is: A solvent is polar if it has a. Is Acetone Polar Protic.
From www.chegg.com
Solved 1. Acetone is a polar aprotic solvent (structure Is Acetone Polar Protic In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly. Our polar aprotic solvent will be acetone. Polar aprotic solvents tend to host sn2 reactions. Although their dielectric constant are. In this example,. Is Acetone Polar Protic.
From www.congress-intercultural.eu
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents, 41 OFF Is Acetone Polar Protic Acetone is a polar aprotic solvent. In this example, we will use an alkyl halide (electrophile) and kf. Although their dielectric constant are. Polar aprotic solvents tend to host sn2 reactions. The leaving group then breaks away. Our polar aprotic solvent will be acetone. The general guideline for solvents regarding nucleophilic substitution reaction is: S n 1 reactions are favored. Is Acetone Polar Protic.
From socratic.org
Polar Protic, Polar Aprotic and NonPolar Solvents Organic Chemistry Is Acetone Polar Protic Acetone is a polar aprotic solvent. A solvent is polar if it has a dipole moment greater than 1.6 d and a dielectric constant greater. Our polar aprotic solvent will be acetone. In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; Although their dielectric constant are. 23 rows however, acetone is still considered a. Is Acetone Polar Protic.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Is Acetone Polar Protic S n 1 reactions are favored by polar protic solvents (h 2 o, roh etc), and usually are solvolysis. In this example, we will use an alkyl halide (electrophile) and kf. Our polar aprotic solvent will be acetone. Both are polar but acn is a polar aprotic and metanol a polar protic. 23 rows however, acetone is still considered a. Is Acetone Polar Protic.
From www.coursehero.com
[Solved] 8. (5 pts. ) We understand phenomena such as solubility in Is Acetone Polar Protic Our polar aprotic solvent will be acetone. In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; The leaving group then breaks away. Polar aprotic solvents tend to host sn2 reactions. The general guideline for solvents regarding nucleophilic substitution reaction is: 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact. Is Acetone Polar Protic.
From www.gizmoplans.com
Is Acetone Polar? Properties, Uses, Molecular Structure Is Acetone Polar Protic S n 1 reactions are favored by polar protic solvents (h 2 o, roh etc), and usually are solvolysis. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Although their dielectric constant are. The general guideline for solvents regarding nucleophilic substitution reaction is: Polar aprotic solvents. Is Acetone Polar Protic.
From answerlibaccuses.z21.web.core.windows.net
Acetone Is Polar Protic Or Aprotic Is Acetone Polar Protic 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Polar aprotic solvents tend to host sn2 reactions. In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; Acetone is a polar aprotic solvent. The general guideline for solvents regarding nucleophilic substitution. Is Acetone Polar Protic.
From psiberg.com
Protic vs Aprotic Solvents (with Examples) PSIBERG Is Acetone Polar Protic Our polar aprotic solvent will be acetone. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. The general guideline for solvents regarding nucleophilic substitution reaction is: Acetone is a polar aprotic solvent. Both are polar but acn is a polar aprotic and metanol a polar protic.. Is Acetone Polar Protic.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Is Acetone Polar Protic The leaving group then breaks away. The general guideline for solvents regarding nucleophilic substitution reaction is: In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; Our polar aprotic solvent will be acetone. A solvent is polar if it has a dipole moment greater than 1.6 d and a dielectric constant greater. 23 rows however,. Is Acetone Polar Protic.
From animalia-life.club
Acetone Lewis Structure With Polarity Is Acetone Polar Protic Our polar aprotic solvent will be acetone. The general guideline for solvents regarding nucleophilic substitution reaction is: 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and. Is Acetone Polar Protic.
From kandicea-ethics.blogspot.com
Polar Protic Solvents Meaning / Tryambak Srivastava On Twitter Polar Is Acetone Polar Protic In this example, we will use an alkyl halide (electrophile) and kf. Although their dielectric constant are. S n 1 reactions are favored by polar protic solvents (h 2 o, roh etc), and usually are solvolysis. Our polar aprotic solvent will be acetone. The leaving group then breaks away. Acetone is a polar aprotic solvent. The general guideline for solvents. Is Acetone Polar Protic.
From www.numerade.com
SOLVED Question 7 3 pts 7. Which of the following solvents could be Is Acetone Polar Protic Polar aprotic solvents tend to host sn2 reactions. Our polar aprotic solvent will be acetone. In sn2 reactions, the nucleophile attacks the electrophile while the leaving group is still attached; Acetone is a polar aprotic solvent. The general guideline for solvents regarding nucleophilic substitution reaction is: 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact. Is Acetone Polar Protic.
From www.pinterest.co.kr
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Organic Is Acetone Polar Protic The general guideline for solvents regarding nucleophilic substitution reaction is: Acetone is a polar aprotic solvent. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Our polar aprotic solvent will be acetone. Although their dielectric constant are. In sn2 reactions, the nucleophile attacks the electrophile while. Is Acetone Polar Protic.
From www.pearson.com
The difference between protic vs. aprotic solvents. Pearson+ Channels Is Acetone Polar Protic Acetone is a polar aprotic solvent. Polar aprotic solvents tend to host sn2 reactions. In this example, we will use an alkyl halide (electrophile) and kf. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. S n 1 reactions are favored by polar protic solvents (h. Is Acetone Polar Protic.
From answerlibaccuses.z21.web.core.windows.net
Acetone Is Polar Protic Or Aprotic Is Acetone Polar Protic S n 1 reactions are favored by polar protic solvents (h 2 o, roh etc), and usually are solvolysis. Both are polar but acn is a polar aprotic and metanol a polar protic. Our polar aprotic solvent will be acetone. Polar aprotic solvents tend to host sn2 reactions. A solvent is polar if it has a dipole moment greater than. Is Acetone Polar Protic.
From www.chegg.com
Solved Which of these solvents are polar protic? Choose all Is Acetone Polar Protic A solvent is polar if it has a dipole moment greater than 1.6 d and a dielectric constant greater. The general guideline for solvents regarding nucleophilic substitution reaction is: 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. In sn2 reactions, the nucleophile attacks the electrophile. Is Acetone Polar Protic.