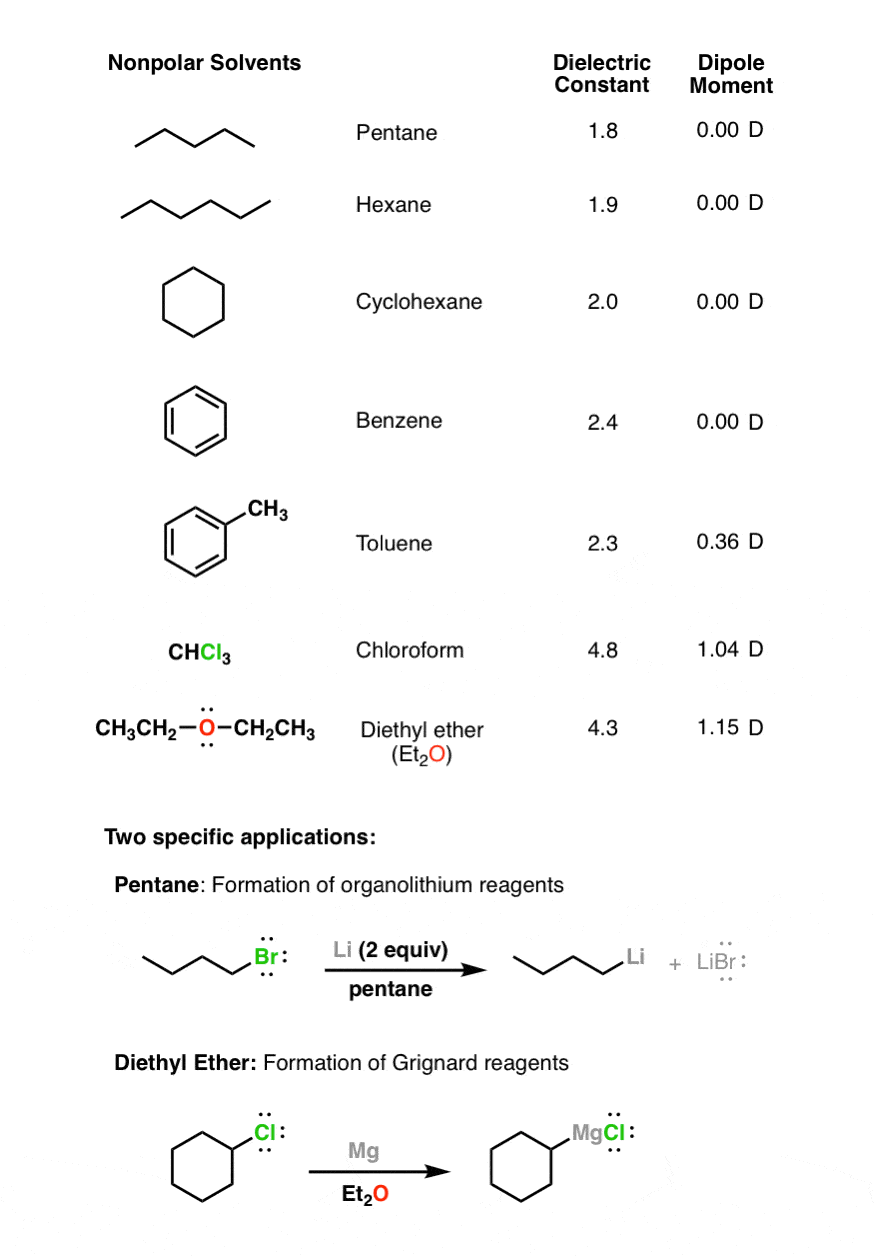
Is Acetone A Polar Protic Solvent . Is acetone a protic or an aprotic polar solvent? Polar solvents are best for dissolving polar reactants (such as ions); Polar solvents contain bonds between atoms with very different electronegativities,. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Learn about its chemical composition, applications, and how it differs. Nonpolar solvents are best for. Protic solvents are polar solvents that contain hydrogen atoms bonded to electronegative atoms, such as water and alcohol. Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. How do polar protic solvents stabilize the chloride and bromide ions? 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. So we can say that acetone is an aprotic polar solvent. Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding.
from julianajoysgraves.blogspot.com
Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. Protic solvents are polar solvents that contain hydrogen atoms bonded to electronegative atoms, such as water and alcohol. Learn about its chemical composition, applications, and how it differs. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. How do polar protic solvents stabilize the chloride and bromide ions? Nonpolar solvents are best for. Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. Polar solvents contain bonds between atoms with very different electronegativities,. Is acetone a protic or an aprotic polar solvent?
Acetone Polar or Nonpolar Solvent JulianajoysGraves
Is Acetone A Polar Protic Solvent Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Protic solvents are polar solvents that contain hydrogen atoms bonded to electronegative atoms, such as water and alcohol. Polar solvents are best for dissolving polar reactants (such as ions); 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. Learn about its chemical composition, applications, and how it differs. Nonpolar solvents are best for. So we can say that acetone is an aprotic polar solvent. Polar solvents contain bonds between atoms with very different electronegativities,. Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. How do polar protic solvents stabilize the chloride and bromide ions? Is acetone a protic or an aprotic polar solvent?
From mungfali.com
Polar Protic Solvent Examples Is Acetone A Polar Protic Solvent Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. Polar solvents contain bonds between atoms with very different electronegativities,. Nonpolar solvents are best for. How do polar protic solvents stabilize the chloride and bromide ions? Is acetone a protic or an aprotic polar solvent? Learn about its chemical composition,. Is Acetone A Polar Protic Solvent.
From chemistrytalk.org
Polar Protic and Aprotic Solvents ChemTalk Is Acetone A Polar Protic Solvent So we can say that acetone is an aprotic polar solvent. Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Polar solvents contain bonds between atoms. Is Acetone A Polar Protic Solvent.
From organicchemistoncall.com
SN2 versus SN1 Organic Chemist On Call Is Acetone A Polar Protic Solvent 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. Protic solvents are polar solvents that contain hydrogen atoms bonded to electronegative atoms, such as water and alcohol. Polar solvents are best. Is Acetone A Polar Protic Solvent.
From www.chegg.com
Solved 9. Which of the following is a polar protic solvent? Is Acetone A Polar Protic Solvent 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Polar solvents are best for dissolving polar reactants (such as ions); 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. How do polar protic solvents stabilize the. Is Acetone A Polar Protic Solvent.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Is Acetone A Polar Protic Solvent Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. Learn about its chemical composition, applications, and how it differs. Polar solvents are best for dissolving polar reactants (such as ions); 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. 23. Is Acetone A Polar Protic Solvent.
From maryi-pubic.blogspot.com
Polar Aprotic Solvents Meaning / Nucleophilic Substitution And Is Acetone A Polar Protic Solvent Polar solvents contain bonds between atoms with very different electronegativities,. Nonpolar solvents are best for. Polar solvents are best for dissolving polar reactants (such as ions); How do polar protic solvents stabilize the chloride and bromide ions? Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. Protic solvents are. Is Acetone A Polar Protic Solvent.
From byjus.com
14. Polar protic and polar aprotic solvents Explain Is Acetone A Polar Protic Solvent How do polar protic solvents stabilize the chloride and bromide ions? Protic solvents are polar solvents that contain hydrogen atoms bonded to electronegative atoms, such as water and alcohol. Polar solvents contain bonds between atoms with very different electronegativities,. Is acetone a protic or an aprotic polar solvent? Nonpolar solvents are best for. Polar solvents are best for dissolving polar. Is Acetone A Polar Protic Solvent.
From www.difference101.com
Protic vs Aprotic Solvents 11 Key Differences To Know Difference 101 Is Acetone A Polar Protic Solvent How do polar protic solvents stabilize the chloride and bromide ions? Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. Nonpolar solvents are best for. Polar solvents are best for dissolving polar reactants (such as ions); Protic solvents are polar solvents that contain hydrogen atoms bonded to electronegative atoms, such. Is Acetone A Polar Protic Solvent.
From www.numerade.com
SOLVEDQuestion 34 (1 point) Example of a polar protic solvent is EtOH Is Acetone A Polar Protic Solvent Learn about its chemical composition, applications, and how it differs. Polar solvents are best for dissolving polar reactants (such as ions); 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. Polar solvents contain bonds between atoms with very different electronegativities,. 23 rows however, acetone is still considered a polar aprotic. Is Acetone A Polar Protic Solvent.
From socratic.org
Polar Protic, Polar Aprotic and NonPolar Solvents Organic Chemistry Is Acetone A Polar Protic Solvent Polar solvents contain bonds between atoms with very different electronegativities,. Learn about its chemical composition, applications, and how it differs. How do polar protic solvents stabilize the chloride and bromide ions? 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. Nonpolar solvents are best for. 23 rows however, acetone is. Is Acetone A Polar Protic Solvent.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID375520 Is Acetone A Polar Protic Solvent Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. How do polar protic solvents stabilize the chloride and bromide ions? Learn about its chemical composition, applications, and how it differs. Protic solvents are polar solvents that contain hydrogen atoms bonded to electronegative atoms, such as water and alcohol. 23 rows. Is Acetone A Polar Protic Solvent.
From mungfali.com
Protic And Aprotic Solvents Is Acetone A Polar Protic Solvent Nonpolar solvents are best for. Is acetone a protic or an aprotic polar solvent? Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Polar solvents are. Is Acetone A Polar Protic Solvent.
From chem-net.blogspot.com
Solvent Effects and SN2 and SN1 reactions Nucleophilic Substitution Is Acetone A Polar Protic Solvent So we can say that acetone is an aprotic polar solvent. Polar solvents are best for dissolving polar reactants (such as ions); How do polar protic solvents stabilize the chloride and bromide ions? Polar solvents contain bonds between atoms with very different electronegativities,. Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen. Is Acetone A Polar Protic Solvent.
From answerlibaccuses.z21.web.core.windows.net
Acetone Is Polar Protic Or Aprotic Is Acetone A Polar Protic Solvent Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. How do polar protic solvents stabilize the chloride and bromide ions? 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Protic solvents are polar solvents that. Is Acetone A Polar Protic Solvent.
From www.chegg.com
Solved Which of these solvents are polar protic? Choose all Is Acetone A Polar Protic Solvent Polar solvents are best for dissolving polar reactants (such as ions); Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Polar solvents contain bonds between atoms. Is Acetone A Polar Protic Solvent.
From chemawareness.blogspot.com
Chem Awareness Classification of Solvent`s Types of Solvents Examples, Is Acetone A Polar Protic Solvent Polar solvents are best for dissolving polar reactants (such as ions); Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. How do polar protic solvents stabilize the chloride and bromide ions? Polar solvents contain bonds between atoms with very different electronegativities,. 23 rows learn about the properties and characteristics of. Is Acetone A Polar Protic Solvent.
From mungfali.com
Polar Protic Solvent Examples Is Acetone A Polar Protic Solvent 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Is acetone a protic or an aprotic polar solvent? Learn about its chemical composition, applications, and how it differs. Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and. Is Acetone A Polar Protic Solvent.
From edu.svet.gob.gt
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Is Acetone A Polar Protic Solvent Polar solvents are best for dissolving polar reactants (such as ions); 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. Learn how to classify acetone as a polar aprotic solvent based. Is Acetone A Polar Protic Solvent.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Is Acetone A Polar Protic Solvent Is acetone a protic or an aprotic polar solvent? Polar solvents are best for dissolving polar reactants (such as ions); So we can say that acetone is an aprotic polar solvent. How do polar protic solvents stabilize the chloride and bromide ions? 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively. Is Acetone A Polar Protic Solvent.
From julianajoysgraves.blogspot.com
Acetone Polar or Nonpolar Solvent JulianajoysGraves Is Acetone A Polar Protic Solvent Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. How do polar protic solvents stabilize the chloride and bromide ions? Is acetone a protic or an aprotic polar solvent? Protic solvents are polar solvents that contain hydrogen atoms bonded to electronegative atoms, such as water and alcohol. Polar solvents are. Is Acetone A Polar Protic Solvent.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Is Acetone A Polar Protic Solvent So we can say that acetone is an aprotic polar solvent. Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. Polar solvents are best for dissolving polar reactants (such as. Is Acetone A Polar Protic Solvent.
From www.numerade.com
SOLVED Br NaCN CN acetonitrile A) What is the solvent (polar/nonpolar Is Acetone A Polar Protic Solvent Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. Learn about its chemical composition, applications, and how it differs. 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. How do polar protic solvents stabilize the chloride and bromide ions?. Is Acetone A Polar Protic Solvent.
From www.pinterest.co.kr
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents Organic Is Acetone A Polar Protic Solvent How do polar protic solvents stabilize the chloride and bromide ions? Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. 23 rows however, acetone is still considered a polar aprotic solvent,. Is Acetone A Polar Protic Solvent.
From www.numerade.com
SOLVED Question 7 3 pts 7. Which of the following solvents could be Is Acetone A Polar Protic Solvent So we can say that acetone is an aprotic polar solvent. Polar solvents are best for dissolving polar reactants (such as ions); Protic solvents are polar solvents that contain hydrogen atoms bonded to electronegative atoms, such as water and alcohol. Learn about its chemical composition, applications, and how it differs. Is acetone a protic or an aprotic polar solvent? 23. Is Acetone A Polar Protic Solvent.
From www.chemistrysteps.com
The Role of Solvent in SN1, SN2, E1 and E2 Reactions Chemistry Steps Is Acetone A Polar Protic Solvent Nonpolar solvents are best for. Learn about its chemical composition, applications, and how it differs. Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. 23 rows however, acetone is still. Is Acetone A Polar Protic Solvent.
From chemistrytalk.org
Polar Protic and Aprotic Solvents ChemTalk Is Acetone A Polar Protic Solvent 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. Is acetone a protic or an aprotic polar solvent? Polar solvents contain bonds between atoms with very different electronegativities,. So we can say that acetone is an aprotic polar solvent. Learn how to classify acetone as a polar aprotic solvent based. Is Acetone A Polar Protic Solvent.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID1699463 Is Acetone A Polar Protic Solvent So we can say that acetone is an aprotic polar solvent. How do polar protic solvents stabilize the chloride and bromide ions? Nonpolar solvents are best for. Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. 23 rows learn about the properties and characteristics of polar protic and aprotic solvents,. Is Acetone A Polar Protic Solvent.
From byjus.com
What is polar protic solvent? Is Acetone A Polar Protic Solvent How do polar protic solvents stabilize the chloride and bromide ions? Is acetone a protic or an aprotic polar solvent? Polar solvents are best for dissolving polar reactants (such as ions); Protic solvents are polar solvents that contain hydrogen atoms bonded to electronegative atoms, such as water and alcohol. Polar solvents contain bonds between atoms with very different electronegativities,. So. Is Acetone A Polar Protic Solvent.
From chemistrytalk.org
Polar Protic and Aprotic Solvents ChemTalk Is Acetone A Polar Protic Solvent 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect chemical. Learn about its chemical composition, applications, and how it differs. How do polar protic solvents stabilize the chloride and bromide ions? Is acetone a protic or an aprotic polar solvent? Polar solvents are best for dissolving polar reactants (such as ions);. Is Acetone A Polar Protic Solvent.
From mavink.com
Polarity Chart Of Solvents Is Acetone A Polar Protic Solvent Protic solvents are polar solvents that contain hydrogen atoms bonded to electronegative atoms, such as water and alcohol. Polar solvents contain bonds between atoms with very different electronegativities,. Polar solvents are best for dissolving polar reactants (such as ions); 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not. Is Acetone A Polar Protic Solvent.
From landynfinpitts.blogspot.com
Acetone Polar or Nonpolar Solvent LandynfinPitts Is Acetone A Polar Protic Solvent Protic solvents are polar solvents that contain hydrogen atoms bonded to electronegative atoms, such as water and alcohol. Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. Is acetone a protic or an aprotic polar solvent? So we can say that acetone is an aprotic polar solvent. 23 rows. Is Acetone A Polar Protic Solvent.
From www.pearson.com
The difference between protic vs. aprotic solvents. Pearson+ Channels Is Acetone A Polar Protic Solvent 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen and carbon. 23 rows learn about the properties and characteristics of polar protic and aprotic solvents, and how they affect. Is Acetone A Polar Protic Solvent.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 The Solvent Master Organic Chemistry Is Acetone A Polar Protic Solvent Nonpolar solvents are best for. Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. Polar solvents are best for dissolving polar reactants (such as ions); Learn about its chemical composition, applications, and how it differs. So we can say that acetone is an aprotic polar solvent. 23 rows however,. Is Acetone A Polar Protic Solvent.
From mungfali.com
Polar Protic Solvent Examples Is Acetone A Polar Protic Solvent 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Learn how to classify acetone as a polar aprotic solvent based on its dipole moment, dielectric constant and hydrogen bonding. Acetone is a polar aprotic solvent with a carbonyl group that forms a dipole bond with oxygen. Is Acetone A Polar Protic Solvent.
From carlynewsturner.blogspot.com
Acetone Polar or Nonpolar Solvent Is Acetone A Polar Protic Solvent Protic solvents are polar solvents that contain hydrogen atoms bonded to electronegative atoms, such as water and alcohol. Polar solvents contain bonds between atoms with very different electronegativities,. 23 rows however, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less. Polar solvents are best for dissolving polar reactants (such. Is Acetone A Polar Protic Solvent.