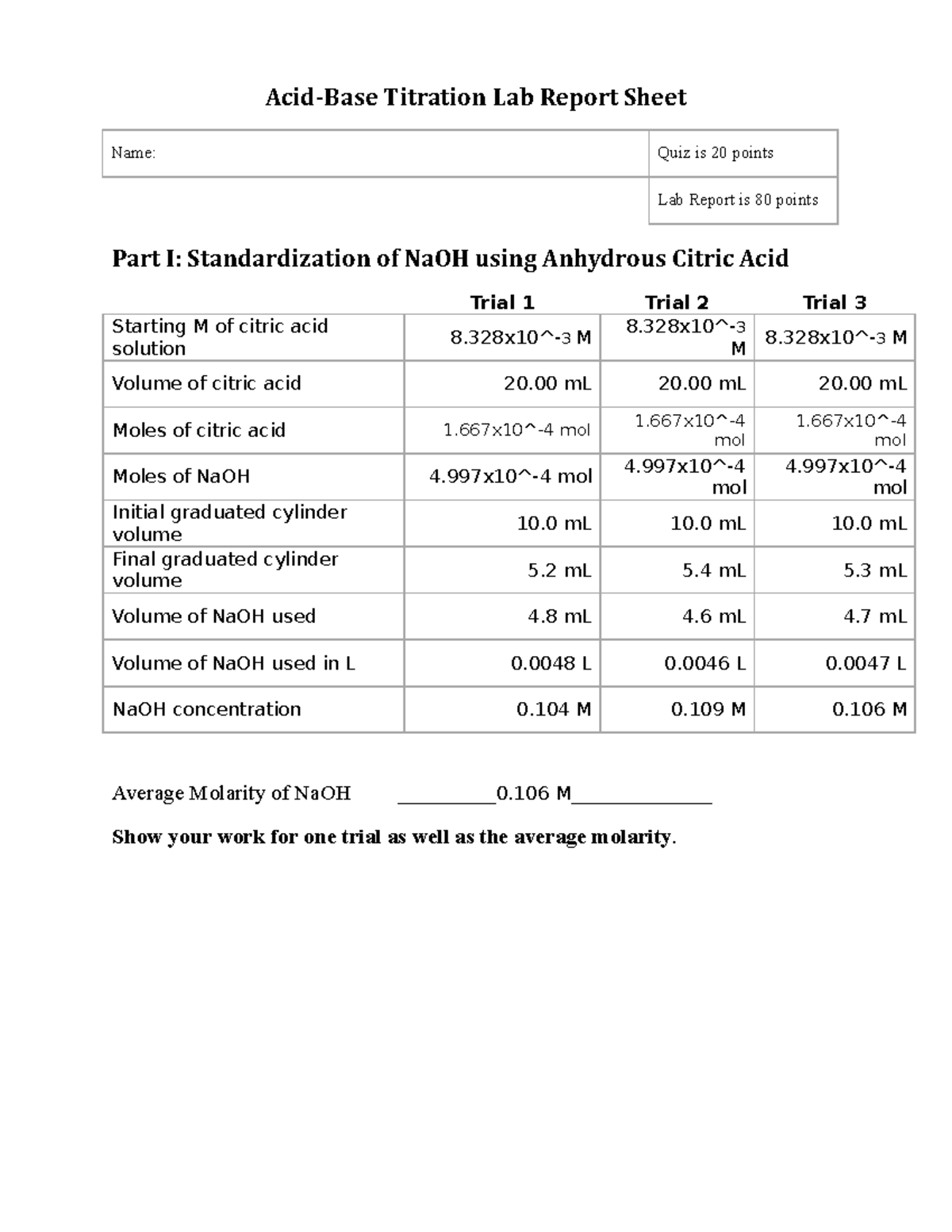
Weak Acid Titration Lab Report . You will be able to: If 15 ml of acetic acid is used instead of 5 ml, the determination of the acetic acid concentration would stay the same, since the molarity is the. From the resulting titration curves, you. (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. Titration of a weak acid with a strong base. 2) titration of weak acid (ch 3 cooh) with strong base (naoh). The volume of solution, such as a strong base, required to react with another reagent, such as a weak acid, is termed titration. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Since the acid is weak, its conductivity is relatively low. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh.
from parkermcyrandolph.blogspot.com
(1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. Titration of a weak acid with a strong base. 2) titration of weak acid (ch 3 cooh) with strong base (naoh). The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. Since the acid is weak, its conductivity is relatively low. You will be able to: The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. From the resulting titration curves, you. If 15 ml of acetic acid is used instead of 5 ml, the determination of the acetic acid concentration would stay the same, since the molarity is the.
Acid Base Titration Lab Report ParkermcyRandolph
Weak Acid Titration Lab Report 2) titration of weak acid (ch 3 cooh) with strong base (naoh). The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. You will be able to: The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. From the resulting titration curves, you. Titration of a weak acid with a strong base. (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. 2) titration of weak acid (ch 3 cooh) with strong base (naoh). Since the acid is weak, its conductivity is relatively low. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. If 15 ml of acetic acid is used instead of 5 ml, the determination of the acetic acid concentration would stay the same, since the molarity is the. The volume of solution, such as a strong base, required to react with another reagent, such as a weak acid, is termed titration.
From moniquearesmeza.blogspot.com
Acid Base Titration Lab Report Answers MoniquearesMeza Weak Acid Titration Lab Report You will be able to: 2) titration of weak acid (ch 3 cooh) with strong base (naoh). From the resulting titration curves, you. Titration of a weak acid with a strong base. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. The reaction of the. Weak Acid Titration Lab Report.
From webapi.bu.edu
Discussion acid base titration lab report. Acid Base Titration Lab Weak Acid Titration Lab Report The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below.. Weak Acid Titration Lab Report.
From www.chegg.com
Solved Acid/Base Reactions Weak Acid Titration Lab Data Weak Acid Titration Lab Report The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. 2) titration of weak acid (ch 3 cooh) with strong base (naoh). The volume of solution, such as a strong base, required to react with another reagent, such as a weak acid, is termed titration. The. Weak Acid Titration Lab Report.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Weak Acid Titration Lab Report (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below.. Weak Acid Titration Lab Report.
From www.studocu.com
ChemistryAcidBase Titration Lab AcidBase Titration Lab Raw Data NH Weak Acid Titration Lab Report The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic acid, with a strong base,. Weak Acid Titration Lab Report.
From freecourseherounlocks.net
Lab Report Potentiometric Titration of a Weak Acid.docx 8 San Jose City Weak Acid Titration Lab Report Titration of a weak acid with a strong base. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. You will be able to: Since the acid is weak, its conductivity is relatively low. The titration curve shown in figure 1 is for the titration of. Weak Acid Titration Lab Report.
From www.vrogue.co
Solution Lab 20 Acid Base Titration Studypool vrogue.co Weak Acid Titration Lab Report If 15 ml of acetic acid is used instead of 5 ml, the determination of the acetic acid concentration would stay the same, since the molarity is the. From the resulting titration curves, you. Since the acid is weak, its conductivity is relatively low. 2) titration of weak acid (ch 3 cooh) with strong base (naoh). (1) determine the hydrogen. Weak Acid Titration Lab Report.
From studylib.net
Titration Lab Weak Acid Titration Lab Report Titration of a weak acid with a strong base. If 15 ml of acetic acid is used instead of 5 ml, the determination of the acetic acid concentration would stay the same, since the molarity is the. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m. Weak Acid Titration Lab Report.
From haileeqohicks.blogspot.com
Acid Base Titration Experiment Report HaileeqoHicks Weak Acid Titration Lab Report The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. If 15 ml of acetic acid is used instead of 5 ml, the determination of the acetic acid concentration would stay the same, since the molarity is the. The reaction of the weak acid, acetic acid,. Weak Acid Titration Lab Report.
From www.studocu.com
Unit 5 Weak AcidBase Titration Lab Report Weak AcidBase Titration Weak Acid Titration Lab Report If 15 ml of acetic acid is used instead of 5 ml, the determination of the acetic acid concentration would stay the same, since the molarity is the. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. (1) determine the hydrogen ion concentration of a. Weak Acid Titration Lab Report.
From www.studocu.com
Lab ReportTitration of weak acid Exp 18 Analysis of a Solid Mixture Weak Acid Titration Lab Report (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. The volume of solution, such as a strong base, required to react with another reagent, such as a weak acid, is termed titration. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch. Weak Acid Titration Lab Report.
From www.vrogue.co
Solved Report Sheet Lab Acid Base Titration 20 A Cheg vrogue.co Weak Acid Titration Lab Report From the resulting titration curves, you. Titration of a weak acid with a strong base. 2) titration of weak acid (ch 3 cooh) with strong base (naoh). The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic acid, with. Weak Acid Titration Lab Report.
From fyoyxafat.blob.core.windows.net
Weak Acid Strong Base Titration Lab Report Discussion at Elizabeth Weak Acid Titration Lab Report The volume of solution, such as a strong base, required to react with another reagent, such as a weak acid, is termed titration. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh. Weak Acid Titration Lab Report.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 12 . Weak Acid Titration Lab Report 2) titration of weak acid (ch 3 cooh) with strong base (naoh). The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. Titration of a weak acid. Weak Acid Titration Lab Report.
From pt.slideshare.net
Lab 3 acid base titration curves and acid_base indicators Weak Acid Titration Lab Report If 15 ml of acetic acid is used instead of 5 ml, the determination of the acetic acid concentration would stay the same, since the molarity is the. You will be able to: Titration of a weak acid with a strong base. From the resulting titration curves, you. (1) determine the hydrogen ion concentration of a weak acid via titration. Weak Acid Titration Lab Report.
From www.vrogue.co
Solved A Weak Acid Strong Base Titration Report Sheet vrogue.co Weak Acid Titration Lab Report 2) titration of weak acid (ch 3 cooh) with strong base (naoh). Titration of a weak acid with a strong base. If 15 ml of acetic acid is used instead of 5 ml, the determination of the acetic acid concentration would stay the same, since the molarity is the. Since the acid is weak, its conductivity is relatively low. From. Weak Acid Titration Lab Report.
From www.vrogue.co
Acid Base Titration Lab Dataclassroom Lab Report Titr vrogue.co Weak Acid Titration Lab Report The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. You will be able to: Titration of a weak acid with a strong base. 2) titration of weak acid. Weak Acid Titration Lab Report.
From www.chegg.com
Solved Titration for Acetic Acid in Vinegar Lab Report Weak Acid Titration Lab Report 2) titration of weak acid (ch 3 cooh) with strong base (naoh). Titration of a weak acid with a strong base. You will be able to: The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. The titration of a weak acid with a strong base. Weak Acid Titration Lab Report.
From mungfali.com
Acid Base Titration Lab Weak Acid Titration Lab Report 2) titration of weak acid (ch 3 cooh) with strong base (naoh). The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh.. Weak Acid Titration Lab Report.
From www.vrogue.co
Acid Base Titration Lab Dataclassroom Lab Report On A vrogue.co Weak Acid Titration Lab Report The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. From the resulting titration curves, you. 2) titration of weak acid (ch 3 cooh) with strong base (naoh). The titration of a weak acid with a strong base involves the direct transfer of protons from the. Weak Acid Titration Lab Report.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Weak Acid Titration Lab Report The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The volume of solution, such as a strong base, required to react with another reagent, such as a weak. Weak Acid Titration Lab Report.
From fyoyxafat.blob.core.windows.net
Weak Acid Strong Base Titration Lab Report Discussion at Elizabeth Weak Acid Titration Lab Report The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. You will be able to: The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. (1) determine the hydrogen ion concentration of a weak acid via titration against a. Weak Acid Titration Lab Report.
From www.studocu.com
P H Weak Acid pH of a weak acid titration lab repor CHEM 111 Studocu Weak Acid Titration Lab Report From the resulting titration curves, you. You will be able to: Since the acid is weak, its conductivity is relatively low. The volume of solution, such as a strong base, required to react with another reagent, such as a weak acid, is termed titration. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen. Weak Acid Titration Lab Report.
From mavink.com
Acid Base Titration Experiment Weak Acid Titration Lab Report (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. The volume of solution, such as a strong base, required to react with another reagent, such as a weak acid, is termed titration. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. The. Weak Acid Titration Lab Report.
From www.vrogue.co
Acid Base Titration Curves Lab Report Titration Of We vrogue.co Weak Acid Titration Lab Report The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. The volume of solution, such as a strong base, required to react with another reagent, such as a weak acid, is termed titration. From the resulting titration curves, you. The reaction of the weak acid, acetic. Weak Acid Titration Lab Report.
From webapi.bu.edu
Discussion acid base titration lab report. Acid Base Titration Lab Weak Acid Titration Lab Report Since the acid is weak, its conductivity is relatively low. If 15 ml of acetic acid is used instead of 5 ml, the determination of the acetic acid concentration would stay the same, since the molarity is the. Titration of a weak acid with a strong base. The titration curve shown in figure 1 is for the titration of 25.00. Weak Acid Titration Lab Report.
From momentumclubs.org
😊 Acid base titration lab report conclusion. Sample Lab Report. 20190125 Weak Acid Titration Lab Report If 15 ml of acetic acid is used instead of 5 ml, the determination of the acetic acid concentration would stay the same, since the molarity is the. From the resulting titration curves, you. 2) titration of weak acid (ch 3 cooh) with strong base (naoh). The volume of solution, such as a strong base, required to react with another. Weak Acid Titration Lab Report.
From www.slideshare.net
Lab 3 acid base titration curves and acid_base indicators Weak Acid Titration Lab Report (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. 2) titration of weak acid (ch 3 cooh) with strong base (naoh). From the resulting titration curves, you. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide. Weak Acid Titration Lab Report.
From www.vrogue.co
Acid Base Titration Curves Lab Report Titration Of We vrogue.co Weak Acid Titration Lab Report 2) titration of weak acid (ch 3 cooh) with strong base (naoh). Titration of a weak acid with a strong base. You will be able to: The volume of solution, such as a strong base, required to react with another reagent, such as a weak acid, is termed titration. The titration of a weak acid with a strong base involves. Weak Acid Titration Lab Report.
From fyoyxafat.blob.core.windows.net
Weak Acid Strong Base Titration Lab Report Discussion at Elizabeth Weak Acid Titration Lab Report 2) titration of weak acid (ch 3 cooh) with strong base (naoh). The volume of solution, such as a strong base, required to react with another reagent, such as a weak acid, is termed titration. Since the acid is weak, its conductivity is relatively low. The titration of a weak acid with a strong base involves the direct transfer of. Weak Acid Titration Lab Report.
From www.vrogue.co
Doc Title Acid Base Titration Preparation Of Standard vrogue.co Weak Acid Titration Lab Report Since the acid is weak, its conductivity is relatively low. You will be able to: The volume of solution, such as a strong base, required to react with another reagent, such as a weak acid, is termed titration. If 15 ml of acetic acid is used instead of 5 ml, the determination of the acetic acid concentration would stay the. Weak Acid Titration Lab Report.
From studylib.net
Titration of an Unknown Weak Acid Weak Acid Titration Lab Report From the resulting titration curves, you. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. Since the acid is weak, its conductivity is relatively low. 2) titration of weak acid (ch 3 cooh) with strong base (naoh). If 15 ml of acetic acid is used. Weak Acid Titration Lab Report.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Weak Acid Titration Lab Report Since the acid is weak, its conductivity is relatively low. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The volume of solution, such as a strong base, required to react with another reagent, such as a weak acid, is termed titration. You will be. Weak Acid Titration Lab Report.
From mungfali.com
Acid Base Titration Lab Weak Acid Titration Lab Report (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. Titration of a weak acid with a strong base. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Since the acid is weak, its conductivity is. Weak Acid Titration Lab Report.
From modernalternativemama.com
lab report titration Weak Acid Titration Lab Report The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3 cooh with 0.100 m naoh. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. 2) titration of weak acid (ch 3 cooh) with strong base (naoh).. Weak Acid Titration Lab Report.