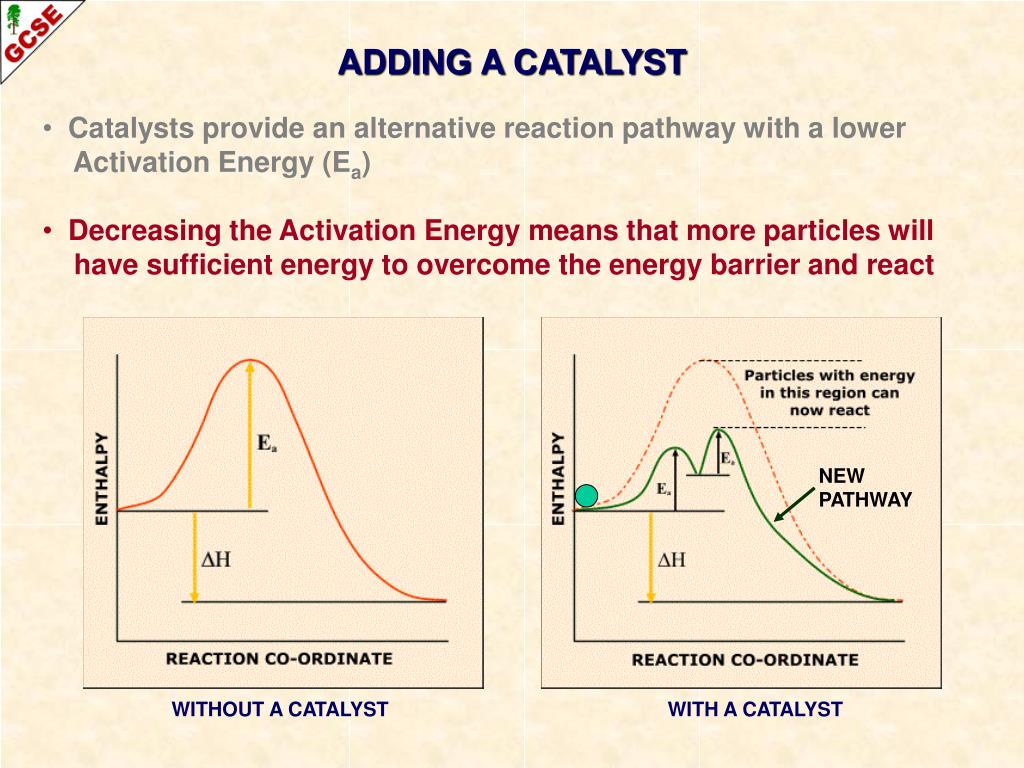
Which Catalysts Lower Activation Energy . learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow. See examples of catalysed reactions, such as. learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how catalysts speed up reactions by providing an alternative route with lower activation energy.
from exoajjasl.blob.core.windows.net
learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. See examples of catalysed reactions, such as. learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow.
A Catalyst Lowers The Activation Energy Of A Reaction From 20 To 10 at
Which Catalysts Lower Activation Energy learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. See examples of catalysed reactions, such as. learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions.
From slideplayer.com
A catalyst lowers activation energy. ppt download Which Catalysts Lower Activation Energy learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts. Which Catalysts Lower Activation Energy.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Which Catalysts Lower Activation Energy See examples of catalysed reactions, such as. learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow. learn how catalysts provide an alternative route for reactions with. Which Catalysts Lower Activation Energy.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Which Catalysts Lower Activation Energy learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism.. Which Catalysts Lower Activation Energy.
From slideplayer.com
Section 6.2 Chemical Reactions ppt download Which Catalysts Lower Activation Energy learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow. learn how. Which Catalysts Lower Activation Energy.
From exoajjasl.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction From 20 To 10 at Which Catalysts Lower Activation Energy learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow.. Which Catalysts Lower Activation Energy.
From studylib.net
Enzymes Lower Activation Energy Which Catalysts Lower Activation Energy learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. See examples of catalysed reactions, such as. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how catalysts lower the activation energy and speed up the rate of. Which Catalysts Lower Activation Energy.
From www.slideserve.com
PPT Enzyme and Catalysis PowerPoint Presentation, free Which Catalysts Lower Activation Energy learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. See examples of catalysed. Which Catalysts Lower Activation Energy.
From quizosteopathy.z4.web.core.windows.net
What Lowers Activation Energy Which Catalysts Lower Activation Energy learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how catalysts. Which Catalysts Lower Activation Energy.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Which Catalysts Lower Activation Energy learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions.. Which Catalysts Lower Activation Energy.
From ceenbrfk.blob.core.windows.net
Catalysts Lower The Activation Energy Required For A Reaction at Elnora Which Catalysts Lower Activation Energy See examples of catalysed reactions, such as. learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how catalysts are used in industry to lower the activation energy and increase the. Which Catalysts Lower Activation Energy.
From dxooagcgl.blob.core.windows.net
How Does The Presence Of A Catalyst Affect The Activation Energy Of A Which Catalysts Lower Activation Energy learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. See examples of. Which Catalysts Lower Activation Energy.
From ceenbrfk.blob.core.windows.net
Catalysts Lower The Activation Energy Required For A Reaction at Elnora Which Catalysts Lower Activation Energy learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how catalysts lower the energy of. Which Catalysts Lower Activation Energy.
From cenkdqaq.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Certain Reaction At 27 at Which Catalysts Lower Activation Energy learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how catalysts increase the rate of. Which Catalysts Lower Activation Energy.
From www.slideserve.com
PPT Enzymes (Part I) PowerPoint Presentation, free download ID2525494 Which Catalysts Lower Activation Energy learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. learn how catalysts. Which Catalysts Lower Activation Energy.
From ceupgijk.blob.core.windows.net
A Catalyst Lowers Activation Energy By at Maud Keen blog Which Catalysts Lower Activation Energy learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that. Which Catalysts Lower Activation Energy.
From ceenbrfk.blob.core.windows.net
Catalysts Lower The Activation Energy Required For A Reaction at Elnora Which Catalysts Lower Activation Energy learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn. Which Catalysts Lower Activation Energy.
From slidetodoc.com
UNIT 1 INTRODUCING BIOLOGY Chapter 2 Chemistry of Which Catalysts Lower Activation Energy learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow. learn. Which Catalysts Lower Activation Energy.
From cenkdqaq.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Certain Reaction At 27 at Which Catalysts Lower Activation Energy learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how catalysts. Which Catalysts Lower Activation Energy.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Which Catalysts Lower Activation Energy learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow. learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how. Which Catalysts Lower Activation Energy.
From byjus.com
Activation Energy Which Catalysts Lower Activation Energy learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. See examples of catalysed reactions, such as. learn how catalysts lower the energy of activation ea by providing a pathway or. Which Catalysts Lower Activation Energy.
From www.slideserve.com
PPT Reaction Rate and Equilibrium PowerPoint Presentation, free Which Catalysts Lower Activation Energy learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. See examples of catalysed reactions, such as. learn how catalysts increase the rate of a reaction by providing an alternative pathway with. Which Catalysts Lower Activation Energy.
From ceupgijk.blob.core.windows.net
A Catalyst Lowers Activation Energy By at Maud Keen blog Which Catalysts Lower Activation Energy learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how. Which Catalysts Lower Activation Energy.
From ceupgijk.blob.core.windows.net
A Catalyst Lowers Activation Energy By at Maud Keen blog Which Catalysts Lower Activation Energy learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. . Which Catalysts Lower Activation Energy.
From slideplayer.com
Chemical Honors Unit ppt download Which Catalysts Lower Activation Energy See examples of catalysed reactions, such as. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. learn how catalysts speed up reactions by providing an alternative route with lower. Which Catalysts Lower Activation Energy.
From slideplayer.com
Section 24 & 25 “Chemical Reactions & Enzymes” ppt download Which Catalysts Lower Activation Energy learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions. Which Catalysts Lower Activation Energy.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID3754310 Which Catalysts Lower Activation Energy learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how catalysts are used in industry. Which Catalysts Lower Activation Energy.
From vdocuments.mx
Catalysts Catalystlowers activation energy of a chemical reaction and Which Catalysts Lower Activation Energy learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how catalysts lower the. Which Catalysts Lower Activation Energy.
From www.slideserve.com
PPT Rates of Reaction & Equilibrium PowerPoint Presentation, free Which Catalysts Lower Activation Energy learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow. learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. . Which Catalysts Lower Activation Energy.
From slideplayer.com
Chapter 24 Chemical Reactions and Enzymes. ppt download Which Catalysts Lower Activation Energy learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow. learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. . Which Catalysts Lower Activation Energy.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Which Catalysts Lower Activation Energy See examples of catalysed reactions, such as. learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how catalysts increase the rate of a reaction by providing an alternative pathway. Which Catalysts Lower Activation Energy.
From www.slideserve.com
PPT Enzymes Biological Catalysts PowerPoint Presentation ID5736986 Which Catalysts Lower Activation Energy learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. See examples of catalysed reactions, such as. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how catalysts speed up reactions by providing an alternative route with lower. Which Catalysts Lower Activation Energy.
From ceugvfxv.blob.core.windows.net
Catalyst Activation Energy Graph at Beard blog Which Catalysts Lower Activation Energy learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how catalysts are used in industry. Which Catalysts Lower Activation Energy.
From cetylqdp.blob.core.windows.net
Describe How Enzymes Lower Activation Energy at Patricia Chambers blog Which Catalysts Lower Activation Energy See examples of catalysed reactions, such as. learn how catalysts lower the energy of activation ea by providing a pathway or transition state for reactions that follow. learn how catalysts are used in industry to lower the activation energy and increase the rate of reactions. learn how a catalyst can lower the activation energy of a reaction. Which Catalysts Lower Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Which Catalysts Lower Activation Energy learn how a catalyst can lower the activation energy of a reaction and increase the rate of successful collisions. See examples of catalysed reactions, such as. learn how catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. learn how catalysts speed up reactions by providing an alternative route with lower. Which Catalysts Lower Activation Energy.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Which Catalysts Lower Activation Energy learn how catalysts lower the activation energy and speed up the rate of reactions by providing an alternate reaction mechanism. learn how catalysts provide an alternative route for reactions with lower activation energy and higher rates. See examples of catalysed reactions, such as. learn how a catalyst can lower the activation energy of a reaction and increase. Which Catalysts Lower Activation Energy.