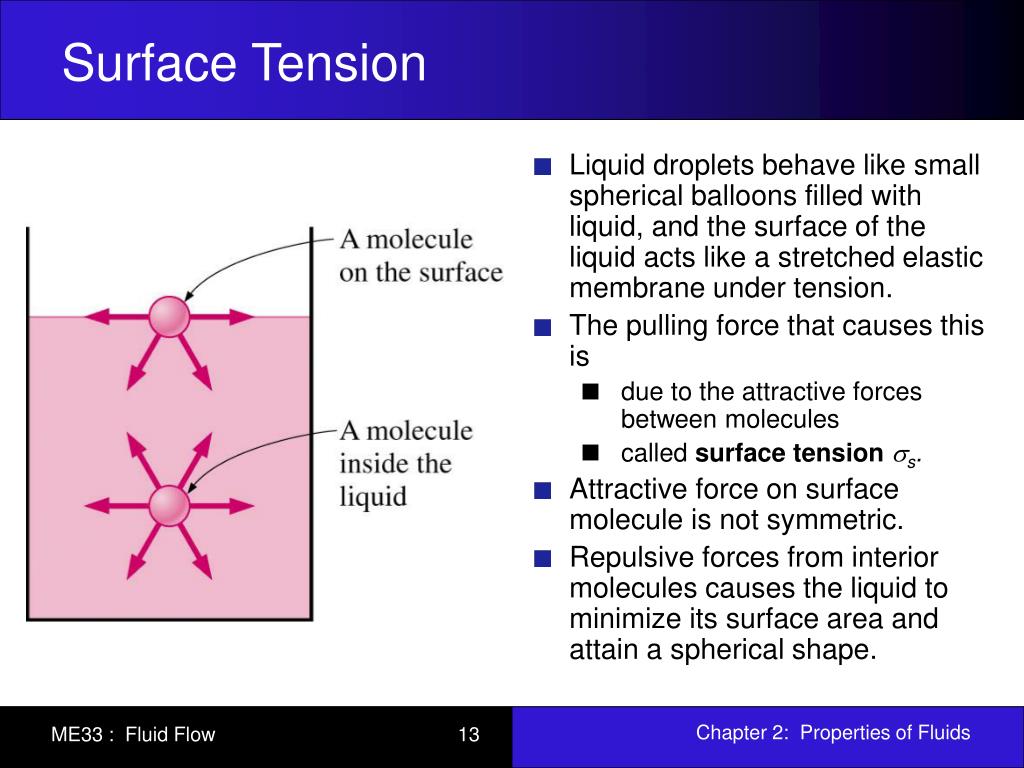
How Does Surface Tension Take Place . Equivalently, it can be stated as surface energy in ergs. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. A water molecule in the middle of. How does polarity affect it. what is surface tension. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Water molecules are attracted to each other by intermolecular forces like hydrogen bonds. This term is typically used only when the liquid surface is in contact with gas (such as the air). Learn the surface tension of water. Check out a few diagrams. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet.
from www.slideserve.com
surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Equivalently, it can be stated as surface energy in ergs. Learn the surface tension of water. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. what is surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Check out a few diagrams. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. A water molecule in the middle of. This term is typically used only when the liquid surface is in contact with gas (such as the air).
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free
How Does Surface Tension Take Place Check out a few diagrams. Learn the surface tension of water. This term is typically used only when the liquid surface is in contact with gas (such as the air). A water molecule in the middle of. Equivalently, it can be stated as surface energy in ergs. Water molecules are attracted to each other by intermolecular forces like hydrogen bonds. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Check out a few diagrams. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. How does polarity affect it. what is surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet.
From www.youtube.com
Surface Tension and Surfactant (Fluid Mechanics Lesson 12) YouTube How Does Surface Tension Take Place How does polarity affect it. Water molecules are attracted to each other by intermolecular forces like hydrogen bonds. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Equivalently, it can be stated as surface energy in ergs. surface tension, property of a liquid surface displayed by its. How Does Surface Tension Take Place.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How Does Surface Tension Take Place surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Check out a few diagrams. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. A water molecule in the middle. How Does Surface Tension Take Place.
From vectormine.com
Surface tension explanation vector illustration diagram VectorMine How Does Surface Tension Take Place How does polarity affect it. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. A water molecule in the middle of. Equivalently, it can be stated as surface energy in ergs. Check out a few diagrams. This term is typically used. How Does Surface Tension Take Place.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does Surface Tension Take Place How does polarity affect it. what is surface tension. This term is typically used only when the liquid surface is in contact with gas (such as the air). A water molecule in the middle of. Learn the surface tension of water. Equivalently, it can be stated as surface energy in ergs. Water molecules are attracted to each other by. How Does Surface Tension Take Place.
From giofsecel.blob.core.windows.net
What Properties Does Surface Tension Itself Affect at Jon blog How Does Surface Tension Take Place Check out a few diagrams. what is surface tension. Water molecules are attracted to each other by intermolecular forces like hydrogen bonds. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. surface tension is typically measured in dynes/cm, the. How Does Surface Tension Take Place.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does Surface Tension Take Place Learn the surface tension of water. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water molecules are attracted to each other by intermolecular forces like hydrogen. How Does Surface Tension Take Place.
From www.pw.live
Surface Tension How Does Surface Tension Take Place surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. surface tension is typically measured in dynes/cm, the force in. How Does Surface Tension Take Place.
From www.slideserve.com
PPT Ch. 2 States of Matter PowerPoint Presentation, free download How Does Surface Tension Take Place what is surface tension. Equivalently, it can be stated as surface energy in ergs. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as. How Does Surface Tension Take Place.
From www.youtube.com
How does Surface Tension work? YouTube How Does Surface Tension Take Place what is surface tension. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. A water molecule in the middle of. Check out a few diagrams. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. How does. How Does Surface Tension Take Place.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint How Does Surface Tension Take Place surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Equivalently, it can be stated as surface energy in ergs. Learn the surface tension of water. A water molecule in the middle of. what is surface tension. surface tension is the energy, or work, required to increase the. How Does Surface Tension Take Place.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical How Does Surface Tension Take Place This term is typically used only when the liquid surface is in contact with gas (such as the air). A water molecule in the middle of. Check out a few diagrams. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, property of a liquid surface. How Does Surface Tension Take Place.
From giozhnupc.blob.core.windows.net
How Is Surface Tension Of Water Important To Life at James Ebert blog How Does Surface Tension Take Place Water molecules are attracted to each other by intermolecular forces like hydrogen bonds. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only when the liquid surface is in contact with gas (such as the air).. How Does Surface Tension Take Place.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL How Does Surface Tension Take Place Equivalently, it can be stated as surface energy in ergs. A water molecule in the middle of. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This term is typically used only when the liquid surface is in contact with gas (such as the air). Check out a few. How Does Surface Tension Take Place.
From hxevucvjl.blob.core.windows.net
How Does Surface Tension Effect Bubbles at Rosemary Herbert blog How Does Surface Tension Take Place Water molecules are attracted to each other by intermolecular forces like hydrogen bonds. How does polarity affect it. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Learn the surface tension of water. Equivalently, it can be stated as surface energy in ergs. This term is typically used. How Does Surface Tension Take Place.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Does Surface Tension Take Place A water molecule in the middle of. what is surface tension. How does polarity affect it. This term is typically used only when the liquid surface is in contact with gas (such as the air). surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as. How Does Surface Tension Take Place.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Does Surface Tension Take Place How does polarity affect it. This term is typically used only when the liquid surface is in contact with gas (such as the air). A water molecule in the middle of. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water molecules are attracted to each other by. How Does Surface Tension Take Place.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid How Does Surface Tension Take Place surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water molecules are attracted to each other by intermolecular forces like. How Does Surface Tension Take Place.
From scienceinfo.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences How Does Surface Tension Take Place what is surface tension. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only when the liquid surface is in contact with gas (such as the air). surface tension is the energy, or work,. How Does Surface Tension Take Place.
From eduinput.com
Surface TensionDefinition, Examples, Causes, And Measurement How Does Surface Tension Take Place surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Water molecules are attracted to each other by intermolecular forces like hydrogen bonds. surface tension is the energy, or work, required to increase the surface area of a liquid due to. How Does Surface Tension Take Place.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID245553 How Does Surface Tension Take Place what is surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Water molecules are attracted to each other by intermolecular forces like hydrogen bonds.. How Does Surface Tension Take Place.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free How Does Surface Tension Take Place A water molecule in the middle of. This term is typically used only when the liquid surface is in contact with gas (such as the air). How does polarity affect it. Water molecules are attracted to each other by intermolecular forces like hydrogen bonds. what is surface tension. Check out a few diagrams. Equivalently, it can be stated as. How Does Surface Tension Take Place.
From giozhnupc.blob.core.windows.net
How Is Surface Tension Of Water Important To Life at James Ebert blog How Does Surface Tension Take Place what is surface tension. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. How does polarity affect it. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. . How Does Surface Tension Take Place.
From fyozfaljz.blob.core.windows.net
How Does Surface Tension Affect Humans at Andrew Rex blog How Does Surface Tension Take Place This term is typically used only when the liquid surface is in contact with gas (such as the air). surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water molecules are attracted to each other by intermolecular forces like hydrogen bonds. Learn the surface tension of water. Check. How Does Surface Tension Take Place.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How Does Surface Tension Take Place How does polarity affect it. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. what is surface tension. This term is typically used only when the. How Does Surface Tension Take Place.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free How Does Surface Tension Take Place How does polarity affect it. what is surface tension. Check out a few diagrams. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. This term is typically used only when the liquid surface is in contact with gas (such as the air). surface tension, property of. How Does Surface Tension Take Place.
From byjus.com
Explain the surface tension phenomenon with examples. How Does Surface Tension Take Place what is surface tension. Learn the surface tension of water. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Check out a few diagrams. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts. How Does Surface Tension Take Place.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL How Does Surface Tension Take Place surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. A water molecule in the middle of. Learn the surface tension of water. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, property of. How Does Surface Tension Take Place.
From civilquery.com
What is Surface Tension Definition, Examples and Tests Civil Query How Does Surface Tension Take Place surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact. How Does Surface Tension Take Place.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog How Does Surface Tension Take Place surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. A water molecule in the middle of. Equivalently, it can be stated as surface energy in ergs. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Learn the. How Does Surface Tension Take Place.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and How Does Surface Tension Take Place A water molecule in the middle of. what is surface tension. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Learn the surface tension of water. Check out a few diagrams. surface tension is a phenomenon in which the surface of a liquid, where the liquid. How Does Surface Tension Take Place.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation How Does Surface Tension Take Place surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Water molecules are attracted to each other by intermolecular forces like hydrogen bonds. A water molecule in the middle of. what is surface tension. This term is typically used only when the liquid surface is in contact with gas. How Does Surface Tension Take Place.
From www.youtube.com
Surface Tension of Water Explained YouTube How Does Surface Tension Take Place How does polarity affect it. what is surface tension. This term is typically used only when the liquid surface is in contact with gas (such as the air). Check out a few diagrams. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Equivalently, it can be stated. How Does Surface Tension Take Place.
From www.merchantnavydecoded.com
What is Surface tension? Why does surface tension occur? Merchant How Does Surface Tension Take Place This term is typically used only when the liquid surface is in contact with gas (such as the air). A water molecule in the middle of. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Learn the surface tension of water. How does polarity affect it. surface tension. How Does Surface Tension Take Place.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Does Surface Tension Take Place surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. How does polarity affect it. Water molecules are attracted to each other by intermolecular forces like hydrogen bonds. This term is typically used only when the liquid surface is in contact with. How Does Surface Tension Take Place.
From www.youtube.com
0V. 59 Where Does Surface Tension Occur and Why? YouTube How Does Surface Tension Take Place Equivalently, it can be stated as surface energy in ergs. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Learn the surface tension of water. what is surface tension. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length. How Does Surface Tension Take Place.