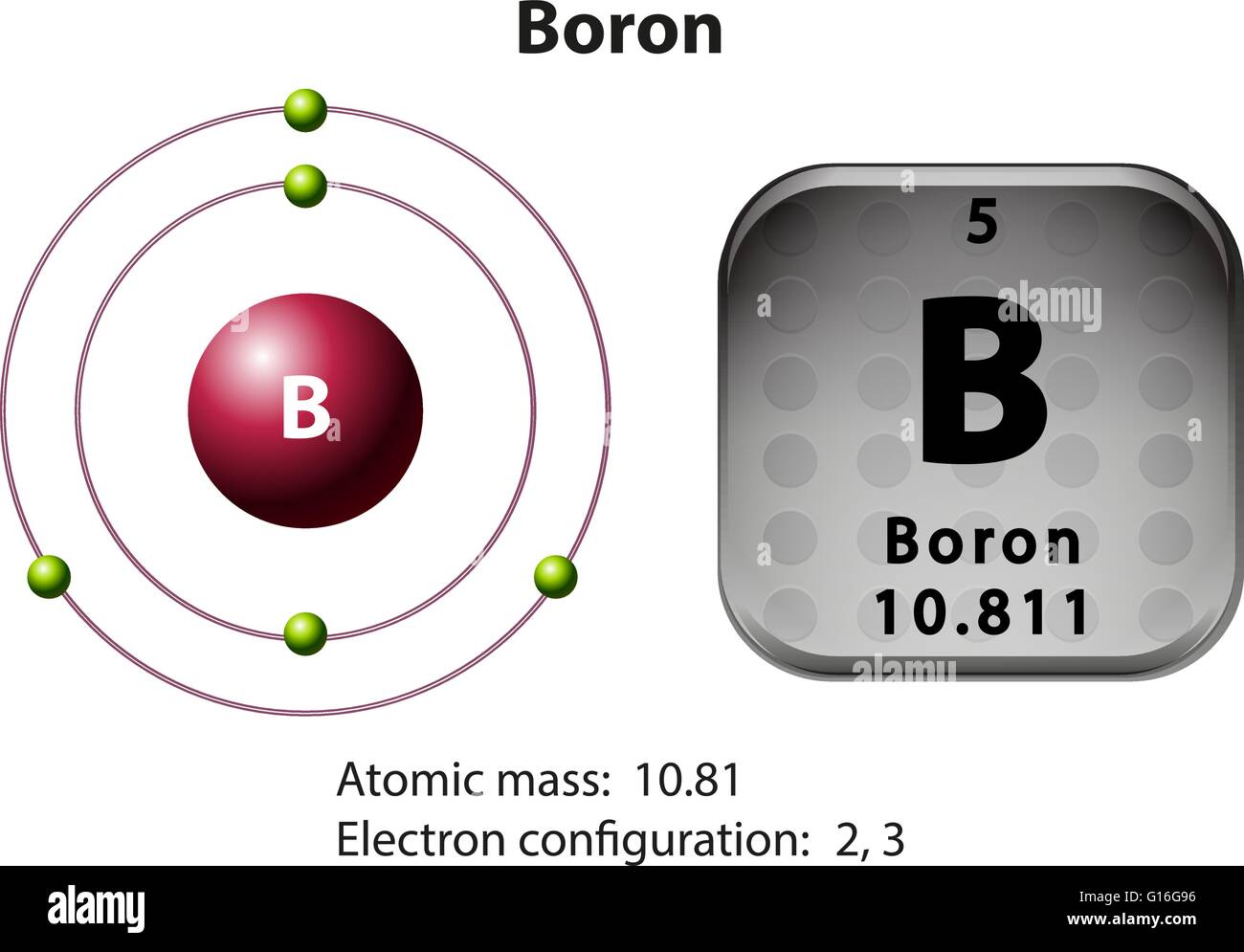
Why Does Boron Want 6 Electrons . The fluorine atoms follow the octet rule, but. Two of them are core electrons and the remaining 3 are valence electrons. the problem with this structure is that boron has an incomplete octet; the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. In b 2 h 6, rather than being a direct replica of its. boron has a charge of 5. the boron shares its three electrons with three fluorine atoms. as it was later discovered, boron was forming cluster compounds to achieve the elusive electron octet. I read in the textbook and it said boron compounds typically have six valence electrons on the central b. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. This is balanced by 5 electrons. It only has six electrons around it.
from www.animalia-life.club
This is balanced by 5 electrons. In b 2 h 6, rather than being a direct replica of its. The fluorine atoms follow the octet rule, but. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. It only has six electrons around it. the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. Two of them are core electrons and the remaining 3 are valence electrons. boron has a charge of 5. the boron shares its three electrons with three fluorine atoms. I read in the textbook and it said boron compounds typically have six valence electrons on the central b.
Boron Protons Neutrons Electrons
Why Does Boron Want 6 Electrons It only has six electrons around it. The fluorine atoms follow the octet rule, but. I read in the textbook and it said boron compounds typically have six valence electrons on the central b. as it was later discovered, boron was forming cluster compounds to achieve the elusive electron octet. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. This is balanced by 5 electrons. boron has a charge of 5. Two of them are core electrons and the remaining 3 are valence electrons. the problem with this structure is that boron has an incomplete octet; the boron shares its three electrons with three fluorine atoms. In b 2 h 6, rather than being a direct replica of its. the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. It only has six electrons around it.
From utedzz.blogspot.com
Periodic Table Boron Atom Periodic Table Timeline Why Does Boron Want 6 Electrons the problem with this structure is that boron has an incomplete octet; as it was later discovered, boron was forming cluster compounds to achieve the elusive electron octet. boron has a charge of 5. the boron shares its three electrons with three fluorine atoms. the octet rule is a bonding theory used to predict the. Why Does Boron Want 6 Electrons.
From www.animalia-life.club
Boron Protons Neutrons Electrons Why Does Boron Want 6 Electrons I read in the textbook and it said boron compounds typically have six valence electrons on the central b. Two of them are core electrons and the remaining 3 are valence electrons. the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. boron has a charge of 5. The fluorine atoms. Why Does Boron Want 6 Electrons.
From www.animalia-life.club
Boron Protons Neutrons Electrons Why Does Boron Want 6 Electrons the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. The fluorine atoms follow the octet rule, but. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. This is balanced by 5 electrons. the problem with this structure. Why Does Boron Want 6 Electrons.
From ar.inspiredpencil.com
Boron Protons Neutrons Electrons Why Does Boron Want 6 Electrons boron has a charge of 5. as it was later discovered, boron was forming cluster compounds to achieve the elusive electron octet. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. the boron shares its three electrons with three fluorine atoms. It only has. Why Does Boron Want 6 Electrons.
From borates.today
Boron Electron Valence Borates Today Why Does Boron Want 6 Electrons as it was later discovered, boron was forming cluster compounds to achieve the elusive electron octet. the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. the problem with this structure is that boron has an incomplete octet; The fluorine atoms follow the octet rule, but. It only has six. Why Does Boron Want 6 Electrons.
From valenceelectrons.com
Boron(B) electron configuration and orbital diagram Why Does Boron Want 6 Electrons I read in the textbook and it said boron compounds typically have six valence electrons on the central b. It only has six electrons around it. This is balanced by 5 electrons. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. In b 2 h 6, rather. Why Does Boron Want 6 Electrons.
From userdatalogarithms.z13.web.core.windows.net
Boron Lewis Diagram Why Does Boron Want 6 Electrons the problem with this structure is that boron has an incomplete octet; as it was later discovered, boron was forming cluster compounds to achieve the elusive electron octet. the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. In b 2 h 6, rather than being a direct replica of. Why Does Boron Want 6 Electrons.
From material-properties.org
Boron Periodic Table and Atomic Properties Why Does Boron Want 6 Electrons the problem with this structure is that boron has an incomplete octet; the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. The fluorine atoms follow the octet rule, but. as it was later discovered, boron was forming cluster compounds to achieve the elusive electron octet.. Why Does Boron Want 6 Electrons.
From www.sciencephoto.com
Boron, atomic structure Stock Image C018/3686 Science Photo Library Why Does Boron Want 6 Electrons the problem with this structure is that boron has an incomplete octet; I read in the textbook and it said boron compounds typically have six valence electrons on the central b. the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. The fluorine atoms follow the octet rule, but. In b. Why Does Boron Want 6 Electrons.
From www.youtube.com
Why does boron only want 6 electrons? YouTube Why Does Boron Want 6 Electrons as it was later discovered, boron was forming cluster compounds to achieve the elusive electron octet. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. The fluorine atoms follow the octet rule, but. I read in the textbook and it said boron compounds typically have six. Why Does Boron Want 6 Electrons.
From www.nagwa.com
Question Video Explaining the Covalent Bonding in Borane Nagwa Why Does Boron Want 6 Electrons The fluorine atoms follow the octet rule, but. the problem with this structure is that boron has an incomplete octet; I read in the textbook and it said boron compounds typically have six valence electrons on the central b. It only has six electrons around it. the boron shares its three electrons with three fluorine atoms. as. Why Does Boron Want 6 Electrons.
From periodictable.me
How To Find The Boron Electron Configuration (B) Why Does Boron Want 6 Electrons The fluorine atoms follow the octet rule, but. I read in the textbook and it said boron compounds typically have six valence electrons on the central b. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. the boron shares its three electrons with three fluorine atoms.. Why Does Boron Want 6 Electrons.
From periodictable.me
How To Find The Electron Configuration For Boron Dynamic Periodic Why Does Boron Want 6 Electrons In b 2 h 6, rather than being a direct replica of its. Two of them are core electrons and the remaining 3 are valence electrons. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. It only has six electrons around it. the problem with this. Why Does Boron Want 6 Electrons.
From guidedehartcosmoramas.z21.web.core.windows.net
Boron Molecular Orbital Diagram Why Does Boron Want 6 Electrons This is balanced by 5 electrons. the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. I read in the textbook and it said boron compounds typically have six valence electrons on the central b. The fluorine atoms follow the octet rule, but. Two of them are core electrons and the remaining. Why Does Boron Want 6 Electrons.
From newtondesk.com
Boron Element With Reaction, Properties, Uses, & Price Periodic Table Why Does Boron Want 6 Electrons This is balanced by 5 electrons. Two of them are core electrons and the remaining 3 are valence electrons. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. The. Why Does Boron Want 6 Electrons.
From mungfali.com
Boron Electron Dot Diagram Why Does Boron Want 6 Electrons This is balanced by 5 electrons. The fluorine atoms follow the octet rule, but. In b 2 h 6, rather than being a direct replica of its. the problem with this structure is that boron has an incomplete octet; It only has six electrons around it. as it was later discovered, boron was forming cluster compounds to achieve. Why Does Boron Want 6 Electrons.
From www.animalia-life.club
Boron Protons Neutrons Electrons Why Does Boron Want 6 Electrons This is balanced by 5 electrons. the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. the problem with this structure is that boron has an incomplete octet; the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. . Why Does Boron Want 6 Electrons.
From mungfali.com
Boron Electron Dot Diagram Why Does Boron Want 6 Electrons Two of them are core electrons and the remaining 3 are valence electrons. the boron shares its three electrons with three fluorine atoms. the problem with this structure is that boron has an incomplete octet; as it was later discovered, boron was forming cluster compounds to achieve the elusive electron octet. I read in the textbook and. Why Does Boron Want 6 Electrons.
From chamotgallery.com
How many protons, neutrons and electrons does boron have? (2022) Why Does Boron Want 6 Electrons the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. The fluorine atoms follow the octet rule, but. It only has six electrons around it. the problem with this structure is that boron has an incomplete octet; Two of them are core electrons and the remaining 3 are valence electrons. I. Why Does Boron Want 6 Electrons.
From iperiodictable.com
How To Find an Valence Boron Electron Configuration (B) Why Does Boron Want 6 Electrons boron has a charge of 5. In b 2 h 6, rather than being a direct replica of its. It only has six electrons around it. This is balanced by 5 electrons. I read in the textbook and it said boron compounds typically have six valence electrons on the central b. the octet rule is a bonding theory. Why Does Boron Want 6 Electrons.
From medium.com
What is Boron? Periodic Table Elements Why Does Boron Want 6 Electrons The fluorine atoms follow the octet rule, but. Two of them are core electrons and the remaining 3 are valence electrons. It only has six electrons around it. This is balanced by 5 electrons. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. boron has a. Why Does Boron Want 6 Electrons.
From quizquadratrix.z21.web.core.windows.net
Boron Atomic Number Of Protons Why Does Boron Want 6 Electrons The fluorine atoms follow the octet rule, but. It only has six electrons around it. In b 2 h 6, rather than being a direct replica of its. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. the problem with this structure is that boron has. Why Does Boron Want 6 Electrons.
From www.animalia-life.club
Boron Protons Neutrons Electrons Why Does Boron Want 6 Electrons the problem with this structure is that boron has an incomplete octet; It only has six electrons around it. the boron shares its three electrons with three fluorine atoms. The fluorine atoms follow the octet rule, but. In b 2 h 6, rather than being a direct replica of its. boron has a charge of 5. This. Why Does Boron Want 6 Electrons.
From www.nuclear-power.com
Boron Atomic Number Atomic Mass Density of Boron Why Does Boron Want 6 Electrons boron has a charge of 5. It only has six electrons around it. This is balanced by 5 electrons. In b 2 h 6, rather than being a direct replica of its. the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. The fluorine atoms follow the octet rule, but. . Why Does Boron Want 6 Electrons.
From www.animalia-life.club
Boron Element Why Does Boron Want 6 Electrons The fluorine atoms follow the octet rule, but. In b 2 h 6, rather than being a direct replica of its. as it was later discovered, boron was forming cluster compounds to achieve the elusive electron octet. Two of them are core electrons and the remaining 3 are valence electrons. boron has a charge of 5. It only. Why Does Boron Want 6 Electrons.
From 9to5science.com
[Solved] Why does Boron only need 6 valence electrons 9to5Science Why Does Boron Want 6 Electrons the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. the problem with this structure is that boron has an incomplete octet; Two of them are core electrons and the remaining 3 are valence electrons. It only has six electrons around it. boron has a charge. Why Does Boron Want 6 Electrons.
From utedzz.blogspot.com
Periodic Table Boron Protons Neutrons Electrons Periodic Table Timeline Why Does Boron Want 6 Electrons boron has a charge of 5. the problem with this structure is that boron has an incomplete octet; The fluorine atoms follow the octet rule, but. the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. In b 2 h 6, rather than being a direct replica of its. . Why Does Boron Want 6 Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Boron Have? Why Does Boron Want 6 Electrons I read in the textbook and it said boron compounds typically have six valence electrons on the central b. the boron shares its three electrons with three fluorine atoms. Two of them are core electrons and the remaining 3 are valence electrons. the octet rule is a bonding theory used to predict the molecular structure of covalently bonded. Why Does Boron Want 6 Electrons.
From www.youtube.com
Does boron have 6 valence electrons? YouTube Why Does Boron Want 6 Electrons as it was later discovered, boron was forming cluster compounds to achieve the elusive electron octet. I read in the textbook and it said boron compounds typically have six valence electrons on the central b. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs of. Two of. Why Does Boron Want 6 Electrons.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID6415669 Why Does Boron Want 6 Electrons It only has six electrons around it. the problem with this structure is that boron has an incomplete octet; the boron shares its three electrons with three fluorine atoms. boron has a charge of 5. The fluorine atoms follow the octet rule, but. the octet rule is a bonding theory used to predict the molecular structure. Why Does Boron Want 6 Electrons.
From www.mediastorehouse.com
Boron, atomic model. Boron has six neutrons (white) Why Does Boron Want 6 Electrons the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. It only has six electrons around it. The fluorine atoms follow the octet rule, but. This is balanced by 5 electrons. the fluorine that shares a double bond with boron has six electrons around it (four from its two lone pairs. Why Does Boron Want 6 Electrons.
From www.animalia-life.club
Boron Protons Neutrons Electrons Why Does Boron Want 6 Electrons as it was later discovered, boron was forming cluster compounds to achieve the elusive electron octet. The fluorine atoms follow the octet rule, but. It only has six electrons around it. Two of them are core electrons and the remaining 3 are valence electrons. the problem with this structure is that boron has an incomplete octet; the. Why Does Boron Want 6 Electrons.
From www.youtube.com
Why does boron only need 6? YouTube Why Does Boron Want 6 Electrons boron has a charge of 5. The fluorine atoms follow the octet rule, but. In b 2 h 6, rather than being a direct replica of its. the octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. This is balanced by 5 electrons. the fluorine that shares a double bond. Why Does Boron Want 6 Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Boron Trihydride? Why Does Boron Want 6 Electrons In b 2 h 6, rather than being a direct replica of its. as it was later discovered, boron was forming cluster compounds to achieve the elusive electron octet. boron has a charge of 5. the problem with this structure is that boron has an incomplete octet; Two of them are core electrons and the remaining 3. Why Does Boron Want 6 Electrons.
From www.bysolar.com
How Solar Cells Work Why Does Boron Want 6 Electrons Two of them are core electrons and the remaining 3 are valence electrons. I read in the textbook and it said boron compounds typically have six valence electrons on the central b. In b 2 h 6, rather than being a direct replica of its. the fluorine that shares a double bond with boron has six electrons around it. Why Does Boron Want 6 Electrons.