Titration Data Definition . Perform and interpret titration calculations. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its concentration.
from www.chegg.com
Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Perform and interpret titration calculations. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its concentration.
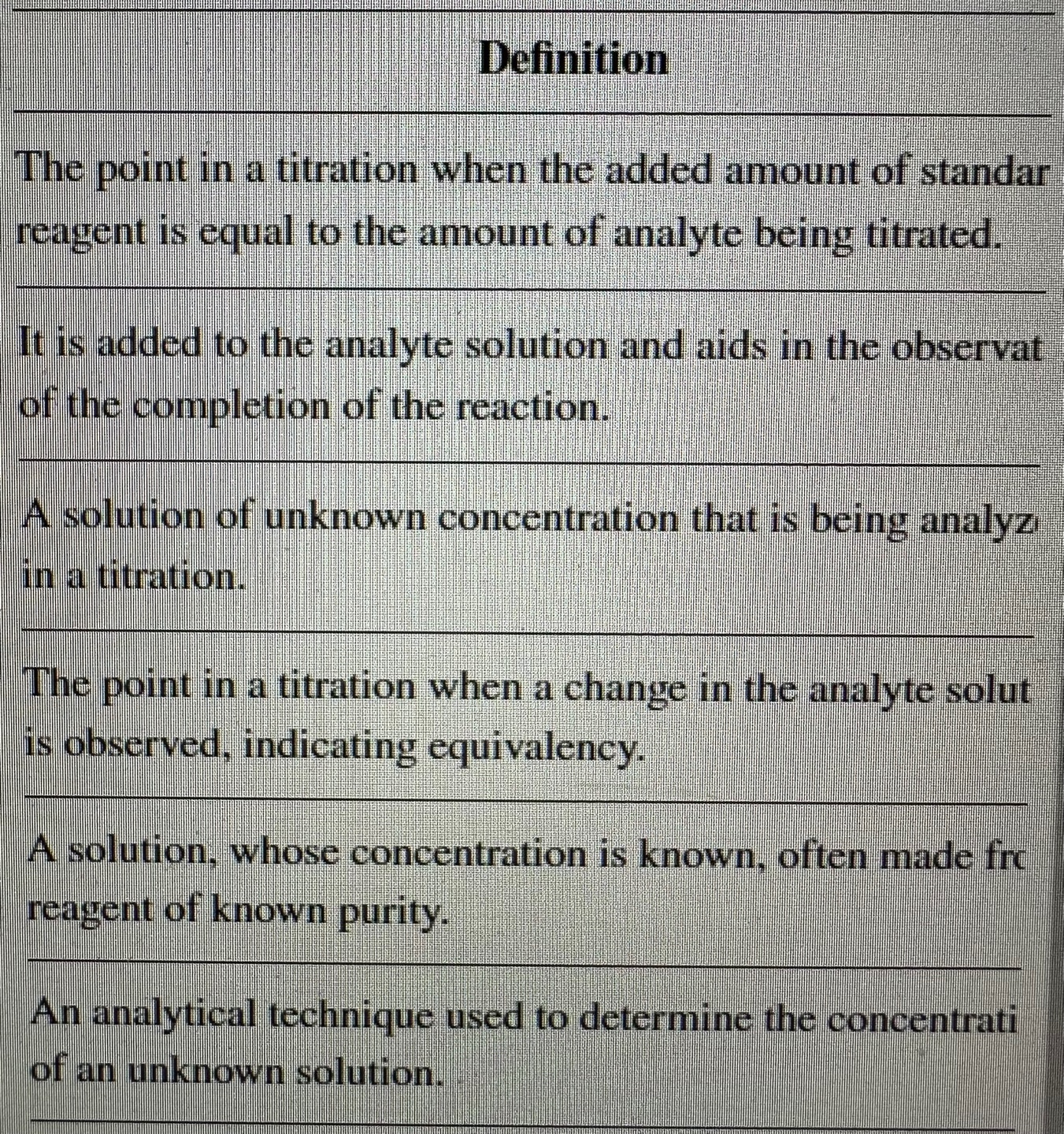
Solved Definition The point in a titration when the added
Titration Data Definition Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. Perform and interpret titration calculations. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its concentration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the.
From facts.net
8 Captivating Facts About AcidBase Titration Titration Data Definition It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known. Titration Data Definition.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Data Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. A titration is an experiment where a volume of a solution of. Titration Data Definition.
From www.numerade.com
SOLVED Define the following terms used in volumetric/titrimetric Titration Data Definition A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its concentration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition. Titration Data Definition.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Data Definition It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration. Titration Data Definition.
From gamesmartz.com
Titration Definition & Image GameSmartz Titration Data Definition Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. It is used in quantitative analytical chemistry to. Titration Data Definition.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Data Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Titration is the process in which one solution is added to another solution such that it reacts. Titration Data Definition.
From www.pw.live
Conductometric titrations Definition, Principle, Theory & Examples PW Titration Data Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is the process in which one solution is added to another. Titration Data Definition.
From www.chegg.com
Solved Definition The point in a titration when the added Titration Data Definition Perform and interpret titration calculations. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. It is used in quantitative analytical chemistry to determine an. Titration Data Definition.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Data Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. Perform and interpret titration calculations. Titration is the process in which one solution is added to another solution such that. Titration Data Definition.
From www.osmosis.org
Introduction to titrations Vídeo & Anatomía Osmosis Titration Data Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition of one solution of a known concentration (called a. Titration Data Definition.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5970774 Titration Data Definition A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration Data Definition.
From mavink.com
Titration Labeled Titration Data Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. Titration Data Definition.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Data Definition Perform and interpret titration calculations. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is the. Titration Data Definition.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Titration Data Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Perform and interpret titration calculations. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. A titration is the process of determining the quantity of one substance by adding measured. Titration Data Definition.
From scienceinfo.com
Titration Definition, 4 Types, Procedure Titration Data Definition A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its concentration. Perform and interpret titration calculations. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration is the slow addition of one solution. Titration Data Definition.
From socratic.org
The "pH" at onehalf the equivalence point in an acidbase titration Titration Data Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. Titration involves the gradual addition of a reagent. Titration Data Definition.
From www.dreamstime.com
Titration Vector Illustration. Labeled Educational Chemistry Process Titration Data Definition A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Perform and interpret titration calculations. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a laboratory technique used. Titration Data Definition.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Data Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. A titration is an experiment where a volume of a solution of known concentration is added to. Titration Data Definition.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Data Definition Perform and interpret titration calculations. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure. Titration Data Definition.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Data Definition Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. A titration is an experiment where a volume. Titration Data Definition.
From mungfali.com
HCl NaOH Titration Titration Data Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Perform and interpret titration calculations. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its concentration. Titration, process of. Titration Data Definition.
From www.slideserve.com
PPT Chem. 31 2/3 Lecture PowerPoint Presentation, free download Titration Data Definition A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. Titration is the slow addition of one solution of a known. Titration Data Definition.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Data Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to. Titration Data Definition.
From www.youtube.com
Titration Definition, steps and formula YouTube Titration Data Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its concentration. A titration is a laboratory. Titration Data Definition.
From www.slideserve.com
PPT Ch 6 Good Titrations PowerPoint Presentation, free download ID Titration Data Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration. Titration Data Definition.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Data Definition It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration. Titration Data Definition.
From www.vrogue.co
Solution Chemistry Acid Base Titration Studypool vrogue.co Titration Data Definition Perform and interpret titration calculations. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. It is used in. Titration Data Definition.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Data Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration. Titration Data Definition.
From chembam.com
Olfactory Titration Experiment Titration Data Definition Perform and interpret titration calculations. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the. Titration Data Definition.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Data Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. A titration is an experiment where a volume of a solution of known concentration is added to a volume of. Titration Data Definition.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Data Definition Perform and interpret titration calculations. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration involves the gradual addition of a reagent of known concentration, known. Titration Data Definition.
From relationshipbetween.com
Difference Between Normality Factor And Titration Error Relationship Titration Data Definition It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its concentration. Perform and interpret titration calculations. Titration is the process in which one solution is added to. Titration Data Definition.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Titration Data Definition Titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its concentration. A titration is the. Titration Data Definition.
From www.numerade.com
SOLVED 5 Use table to record your titration data in the data and Titration Data Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. It is used in quantitative analytical chemistry to determine. Titration Data Definition.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Data Definition A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its concentration. Perform and interpret titration calculations. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is the. Titration Data Definition.