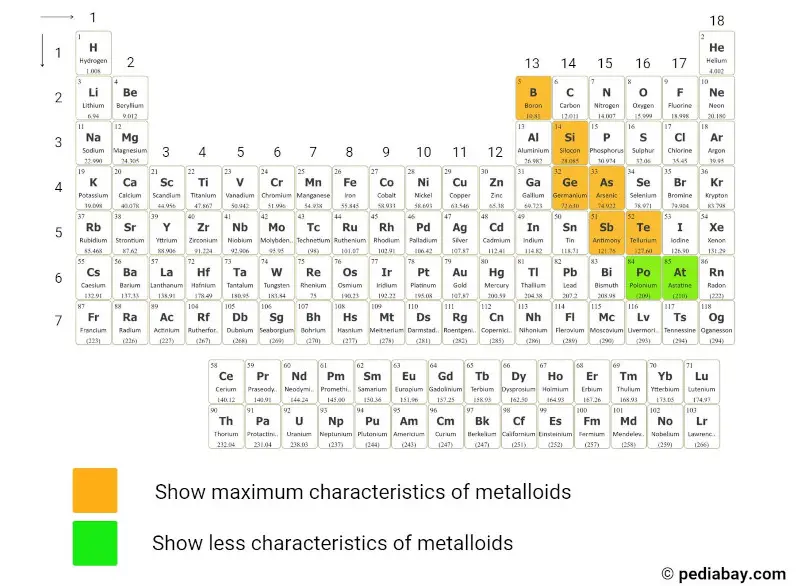
Are Metalloids Hard Or Soft . A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metals have 1 to 3 electrons in the outermost shell of their atoms. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. Good examples of metalloids are elements like boron and silicon that form rather lustrous and flaky substances that look somewhat like the transition. Known for being one of the hardest substances, with a hardness of. For example, mercury is a liquid at room temperature. All metals are hard except sodium and potassium, which are soft and can be cut with a knife. They fall between metals and nonmetals on the periodic table. All metals are hard except sodium and potassium, which are soft and can be cut with a knife. Metals typically have 1 to 3 electrons in the outermost shell of their atoms. The metals in group 1, such as lithium, sodium and potassium, are all soft. The definition of metalloids, as well as the. Metalloids are a class of elements that have properties of both metals and nonmetals. Metals are good conductors because they have free electrons.
from pediabay.com
All metals are hard except sodium and potassium, which are soft and can be cut with a knife. The metals in group 1, such as lithium, sodium and potassium, are all soft. Metals are good conductors because they have free electrons. Metalloids are a class of elements that have properties of both metals and nonmetals. They fall between metals and nonmetals on the periodic table. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. All metals are hard except sodium and potassium, which are soft and can be cut with a knife. For example, mercury is a liquid at room temperature. Metals typically have 1 to 3 electrons in the outermost shell of their atoms. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table.
Metalloids of the Periodic Table Pediabay
Are Metalloids Hard Or Soft The definition of metalloids, as well as the. Known for being one of the hardest substances, with a hardness of. All metals are hard except sodium and potassium, which are soft and can be cut with a knife. All metals are hard except sodium and potassium, which are soft and can be cut with a knife. Good examples of metalloids are elements like boron and silicon that form rather lustrous and flaky substances that look somewhat like the transition. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. They fall between metals and nonmetals on the periodic table. The metals in group 1, such as lithium, sodium and potassium, are all soft. For example, mercury is a liquid at room temperature. Metals have 1 to 3 electrons in the outermost shell of their atoms. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. The definition of metalloids, as well as the. Metals typically have 1 to 3 electrons in the outermost shell of their atoms. Metalloids are a class of elements that have properties of both metals and nonmetals. Metals are good conductors because they have free electrons.
From giovxdujf.blob.core.windows.net
What Groups Are Metalloids In at Doreen Hinojos blog Are Metalloids Hard Or Soft Good examples of metalloids are elements like boron and silicon that form rather lustrous and flaky substances that look somewhat like the transition. Metals are good conductors because they have free electrons. All metals are hard except sodium and potassium, which are soft and can be cut with a knife. A series of six elements called the metalloids separate the. Are Metalloids Hard Or Soft.
From www.xometry.com
Metalloids Properties and Uses Xometry Are Metalloids Hard Or Soft Metals have 1 to 3 electrons in the outermost shell of their atoms. Good examples of metalloids are elements like boron and silicon that form rather lustrous and flaky substances that look somewhat like the transition. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. Metalloids are a class of elements that have properties of both metals and nonmetals.. Are Metalloids Hard Or Soft.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Are Metalloids Hard Or Soft For example, mercury is a liquid at room temperature. Good examples of metalloids are elements like boron and silicon that form rather lustrous and flaky substances that look somewhat like the transition. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Known for being one of the hardest substances, with a. Are Metalloids Hard Or Soft.
From sciencenotes.org
Metalloids Science Notes and Projects Are Metalloids Hard Or Soft All metals are hard except sodium and potassium, which are soft and can be cut with a knife. They fall between metals and nonmetals on the periodic table. The metals in group 1, such as lithium, sodium and potassium, are all soft. Good examples of metalloids are elements like boron and silicon that form rather lustrous and flaky substances that. Are Metalloids Hard Or Soft.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Are Metalloids Hard Or Soft Known for being one of the hardest substances, with a hardness of. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. All metals are hard except sodium and potassium, which are soft and can be cut with a knife. Metals are. Are Metalloids Hard Or Soft.
From thechemistrynotes.com
Metalloids Definition, Properties, Uses, and Applications Are Metalloids Hard Or Soft All metals are hard except sodium and potassium, which are soft and can be cut with a knife. Metalloids are a class of elements that have properties of both metals and nonmetals. For example, mercury is a liquid at room temperature. The metals in group 1, such as lithium, sodium and potassium, are all soft. Known for being one of. Are Metalloids Hard Or Soft.
From scienceinfo.com
Metalloids Definition, Properties, Uses, and Applications Are Metalloids Hard Or Soft Metalloids are a class of elements that have properties of both metals and nonmetals. Known for being one of the hardest substances, with a hardness of. Metals have 1 to 3 electrons in the outermost shell of their atoms. The definition of metalloids, as well as the. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. Metals typically have. Are Metalloids Hard Or Soft.
From www.difference101.com
Metals vs. Nonmetals vs. Metalloids 5 Key Differences, Pros & Cons Are Metalloids Hard Or Soft All metals are hard except sodium and potassium, which are soft and can be cut with a knife. They fall between metals and nonmetals on the periodic table. The definition of metalloids, as well as the. Good examples of metalloids are elements like boron and silicon that form rather lustrous and flaky substances that look somewhat like the transition. All. Are Metalloids Hard Or Soft.
From www.slideserve.com
PPT Metals, Metalloids, and Nonmetals PowerPoint Presentation, free Are Metalloids Hard Or Soft A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Known for being one of the hardest substances, with a hardness of. Metals are good conductors because they have free electrons. Metalloids are a class of elements that have properties of both metals and nonmetals. All metals are hard except sodium and. Are Metalloids Hard Or Soft.
From www.difference101.com
Metals vs. Nonmetals vs. Metalloids 5 Key Differences, Pros & Cons Are Metalloids Hard Or Soft All metals are hard except sodium and potassium, which are soft and can be cut with a knife. Metals have 1 to 3 electrons in the outermost shell of their atoms. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metals typically have 1 to 3 electrons in the outermost shell. Are Metalloids Hard Or Soft.
From slideplayer.com
Properties of Matter Physical Properties ppt download Are Metalloids Hard Or Soft Good examples of metalloids are elements like boron and silicon that form rather lustrous and flaky substances that look somewhat like the transition. They fall between metals and nonmetals on the periodic table. Metals typically have 1 to 3 electrons in the outermost shell of their atoms. Known for being one of the hardest substances, with a hardness of. For. Are Metalloids Hard Or Soft.
From www.slideserve.com
PPT Organizing the Elements Metals Nonmetals and Metalloids Are Metalloids Hard Or Soft The metals in group 1, such as lithium, sodium and potassium, are all soft. All metals are hard except sodium and potassium, which are soft and can be cut with a knife. Metals have 1 to 3 electrons in the outermost shell of their atoms. The definition of metalloids, as well as the. All metals are hard except sodium and. Are Metalloids Hard Or Soft.
From www.worksheetsplanet.com
What are Metalloids? Are Metalloids Hard Or Soft Metals are good conductors because they have free electrons. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. The definition of metalloids, as well as the. Metalloids are a class of elements that have properties of both metals and nonmetals. For example, mercury is a liquid at room temperature. Metals have 1 to 3 electrons in the outermost shell. Are Metalloids Hard Or Soft.
From www.haikudeck.com
Metalloids by Megan Maul Are Metalloids Hard Or Soft Metals have 1 to 3 electrons in the outermost shell of their atoms. Known for being one of the hardest substances, with a hardness of. Metalloids are a class of elements that have properties of both metals and nonmetals. They fall between metals and nonmetals on the periodic table. Metals are good conductors because they have free electrons. A series. Are Metalloids Hard Or Soft.
From newjobs.adda247.com
What are Metalloids? Definition, Properties and Example Are Metalloids Hard Or Soft The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. Known for being one of the hardest substances, with a hardness of. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. They fall between metals and nonmetals on the periodic table. Metals are good conductors because they have free electrons. Metalloids. Are Metalloids Hard Or Soft.
From www.geeksforgeeks.org
Metalloids Definition, Position in Periodic Table, & Properties Are Metalloids Hard Or Soft Metals typically have 1 to 3 electrons in the outermost shell of their atoms. They fall between metals and nonmetals on the periodic table. Known for being one of the hardest substances, with a hardness of. Metals are good conductors because they have free electrons. The metals in group 1, such as lithium, sodium and potassium, are all soft. Metalloids. Are Metalloids Hard Or Soft.
From www.youtube.com
Difference between Metals , Metalloids , and Nonmetals YouTube Are Metalloids Hard Or Soft A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. All metals are hard except sodium and potassium, which are soft and can be cut with a knife. Known for being one of the hardest substances, with a hardness of. The metals in group 1, such as lithium, sodium and potassium, are. Are Metalloids Hard Or Soft.
From pediabay.com
Metalloids of the Periodic Table Pediabay Are Metalloids Hard Or Soft Good examples of metalloids are elements like boron and silicon that form rather lustrous and flaky substances that look somewhat like the transition. They fall between metals and nonmetals on the periodic table. Metals are good conductors because they have free electrons. All metals are hard except sodium and potassium, which are soft and can be cut with a knife.. Are Metalloids Hard Or Soft.
From knordslearning.com
Metalloids Periodic Table (With Images) Are Metalloids Hard Or Soft For example, mercury is a liquid at room temperature. Metalloids are a class of elements that have properties of both metals and nonmetals. The metals in group 1, such as lithium, sodium and potassium, are all soft. They fall between metals and nonmetals on the periodic table. Metals typically have 1 to 3 electrons in the outermost shell of their. Are Metalloids Hard Or Soft.
From www.slideserve.com
PPT THE PERIODIC TABLE PowerPoint Presentation, free download ID Are Metalloids Hard Or Soft A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Known for being one of the hardest substances, with a hardness of. The metals in group 1, such as lithium, sodium and potassium, are all soft. Metalloids are a class of elements that have properties of both metals and nonmetals. All metals. Are Metalloids Hard Or Soft.
From sciencetrends.com
4 Properties Of Metalloids Science Trends Are Metalloids Hard Or Soft Metals have 1 to 3 electrons in the outermost shell of their atoms. All metals are hard except sodium and potassium, which are soft and can be cut with a knife. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metals typically have 1 to 3 electrons in the outermost shell. Are Metalloids Hard Or Soft.
From slideplayer.com
Metals, Nonmetals, and Metalloids ppt download Are Metalloids Hard Or Soft Good examples of metalloids are elements like boron and silicon that form rather lustrous and flaky substances that look somewhat like the transition. Metals are good conductors because they have free electrons. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metalloids are a class of elements that have properties of. Are Metalloids Hard Or Soft.
From edutechspot.com
Metalloids are located where on the periodic table? Here >>> Are Metalloids Hard Or Soft For example, mercury is a liquid at room temperature. Metals typically have 1 to 3 electrons in the outermost shell of their atoms. Metals are good conductors because they have free electrons. They fall between metals and nonmetals on the periodic table. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. Known for being one of the hardest substances,. Are Metalloids Hard Or Soft.
From www.chemistrylearner.com
Metalloids Chemistry Learner Are Metalloids Hard Or Soft Metals have 1 to 3 electrons in the outermost shell of their atoms. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metalloids are a class of elements that have properties of both metals and nonmetals. Good examples of metalloids are. Are Metalloids Hard Or Soft.
From somaap.org
Are metalloids insulators, Conductors and Insulators Are Metalloids Hard Or Soft Metalloids are a class of elements that have properties of both metals and nonmetals. Known for being one of the hardest substances, with a hardness of. Metals typically have 1 to 3 electrons in the outermost shell of their atoms. Metals have 1 to 3 electrons in the outermost shell of their atoms. Metals are good conductors because they have. Are Metalloids Hard Or Soft.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint Are Metalloids Hard Or Soft All metals are hard except sodium and potassium, which are soft and can be cut with a knife. The metals in group 1, such as lithium, sodium and potassium, are all soft. Metalloids are a class of elements that have properties of both metals and nonmetals. For example, mercury is a liquid at room temperature. All metals are hard except. Are Metalloids Hard Or Soft.
From www.slideserve.com
PPT Metals, Metalloids, and Nonmetals PowerPoint Presentation, free Are Metalloids Hard Or Soft A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metalloids are a class of elements that have properties of both metals and nonmetals. Metals have 1 to 3 electrons in the outermost shell of their atoms. They fall between metals and nonmetals on the periodic table. Known for being one of. Are Metalloids Hard Or Soft.
From www.slideserve.com
PPT The Periodic Table of Elements PowerPoint Presentation, free Are Metalloids Hard Or Soft Known for being one of the hardest substances, with a hardness of. Good examples of metalloids are elements like boron and silicon that form rather lustrous and flaky substances that look somewhat like the transition. Metals are good conductors because they have free electrons. A series of six elements called the metalloids separate the metals from the nonmetals in the. Are Metalloids Hard Or Soft.
From sciencenotes.org
5 Examples of Metals, Metalloids, and Nonmetals Are Metalloids Hard Or Soft The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. All metals are hard except sodium and potassium, which are soft and can be cut with a knife. Metalloids are a class of elements that have properties of both metals and nonmetals.. Are Metalloids Hard Or Soft.
From homedeso.vercel.app
Metals And Metalloids On Periodic Table Are Metalloids Hard Or Soft All metals are hard except sodium and potassium, which are soft and can be cut with a knife. Metalloids are a class of elements that have properties of both metals and nonmetals. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium.. Are Metalloids Hard Or Soft.
From www.slideserve.com
PPT Metals, Metalloids, and Nonmetals PowerPoint Presentation, free Are Metalloids Hard Or Soft For example, mercury is a liquid at room temperature. Metals typically have 1 to 3 electrons in the outermost shell of their atoms. Metalloids are a class of elements that have properties of both metals and nonmetals. Known for being one of the hardest substances, with a hardness of. A series of six elements called the metalloids separate the metals. Are Metalloids Hard Or Soft.
From www.difference.wiki
Metals vs. Metalloids What’s the Difference? Are Metalloids Hard Or Soft Good examples of metalloids are elements like boron and silicon that form rather lustrous and flaky substances that look somewhat like the transition. Metals have 1 to 3 electrons in the outermost shell of their atoms. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. The definition of metalloids, as well as the. Metalloids are a class of elements. Are Metalloids Hard Or Soft.
From blog.thepipingmart.com
Metalloids Uses and Properties Are Metalloids Hard Or Soft All metals are hard except sodium and potassium, which are soft and can be cut with a knife. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. Metals have 1 to 3 electrons in the outermost shell of their atoms. Metals are good conductors because they have free electrons. Metals typically have 1 to 3 electrons in the outermost. Are Metalloids Hard Or Soft.
From www.slideserve.com
PPT Chapter 3 Elements, Compounds, and the Periodic Table PowerPoint Are Metalloids Hard Or Soft All metals are hard except sodium and potassium, which are soft and can be cut with a knife. All metals are hard except sodium and potassium, which are soft and can be cut with a knife. Metals have 1 to 3 electrons in the outermost shell of their atoms. A series of six elements called the metalloids separate the metals. Are Metalloids Hard Or Soft.
From www.slideserve.com
PPT The Modern Periodic Table Chapter 6 PowerPoint Presentation, free Are Metalloids Hard Or Soft For example, mercury is a liquid at room temperature. Known for being one of the hardest substances, with a hardness of. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. Metals typically have 1 to 3 electrons in the outermost shell of their atoms. The metals in group 1, such as lithium, sodium and potassium, are all soft. They. Are Metalloids Hard Or Soft.