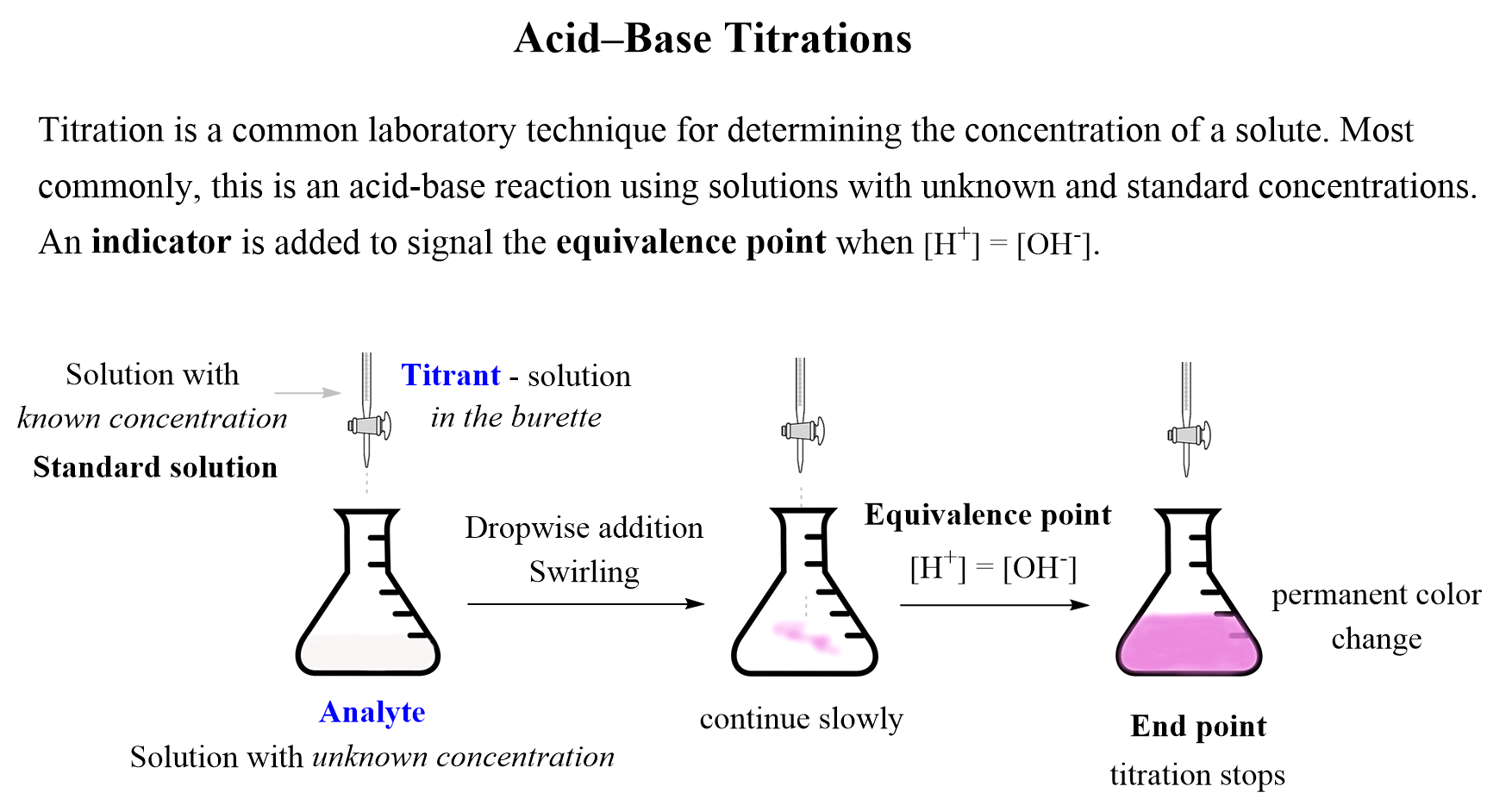
Titration To Determine The Concentration Of An Acid . Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. \ce{naoh}\) is required to reach the end point when titrated. Here is how to perform the calculation. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. Suppose that a titration is performed and \(20.70 \: A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titrating strong acids and strong bases. A titration can be performed with. Titrating a weak acid with a strong base. It is based on the neutralization reaction, where. In a titration experiment, a solution.
from general.chemistrysteps.com
Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. Titrating a weak acid with a strong base. It is based on the neutralization reaction, where. A titration can be performed with. In a titration experiment, a solution. Here is how to perform the calculation.
Strong AcidStrong Base Titrations Chemistry Steps
Titration To Determine The Concentration Of An Acid Titrating a weak acid with a strong base. A titration can be performed with. In a titration experiment, a solution. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. Titrating strong acids and strong bases. Here is how to perform the calculation. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. Titrating a weak acid with a strong base. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: It is based on the neutralization reaction, where.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration To Determine The Concentration Of An Acid Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. In a titration experiment, a solution. It is based on the neutralization reaction, where. Titrating a weak acid with a strong base. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. When we want. Titration To Determine The Concentration Of An Acid.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration To Determine The Concentration Of An Acid Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. Titrating strong acids and strong bases. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. Here is how to perform the calculation. \ce{naoh}\) is required to reach the end point when titrated. Titration is. Titration To Determine The Concentration Of An Acid.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Titration To Determine The Concentration Of An Acid A titration can be performed with. Titrating a weak acid with a strong base. Suppose that a titration is performed and \(20.70 \: Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Here is how to perform the calculation. A chemical reaction is set up between a known volume of. Titration To Determine The Concentration Of An Acid.
From morioh.com
Acid Base Titration Curves PH Calculations Titration To Determine The Concentration Of An Acid Titrating a weak acid with a strong base. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. It is based on the neutralization reaction, where. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In a titration experiment, a solution. Suppose that a. Titration To Determine The Concentration Of An Acid.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration To Determine The Concentration Of An Acid \ce{naoh}\) is required to reach the end point when titrated. Here is how to perform the calculation. Titrating a weak acid with a strong base. It is based on the neutralization reaction, where. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration.. Titration To Determine The Concentration Of An Acid.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Titration To Determine The Concentration Of An Acid A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Titrating strong acids and strong bases. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. \ce{naoh}\) is required to reach the end point. Titration To Determine The Concentration Of An Acid.
From philschatz.com
AcidBase Titrations · Chemistry Titration To Determine The Concentration Of An Acid A titration can be performed with. Titrating a weak acid with a strong base. Suppose that a titration is performed and \(20.70 \: Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. It is based on the neutralization reaction, where. In a titration experiment, a solution. \ce{naoh}\) is required to. Titration To Determine The Concentration Of An Acid.
From socratic.org
How to calculate the concentration of the acid solutions? Socratic Titration To Determine The Concentration Of An Acid Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. In a titration experiment, a solution. \ce{naoh}\) is required to reach the end point when titrated. Titration. Titration To Determine The Concentration Of An Acid.
From theedge.com.hk
Chemistry How To Titration The Edge Titration To Determine The Concentration Of An Acid A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Here is how to perform the calculation. It is based on the neutralization reaction, where. Titrating strong acids and strong bases. \ce{naoh}\) is required to reach the end point when titrated. A titration. Titration To Determine The Concentration Of An Acid.
From www.science-revision.co.uk
Titrations Titration To Determine The Concentration Of An Acid Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titrating a weak acid with a strong base. A titration can be performed with. In a titration experiment, a solution. Titrating strong acids and strong bases. Here is how to perform the calculation. It is based on the neutralization reaction, where.. Titration To Determine The Concentration Of An Acid.
From joixjswcw.blob.core.windows.net
Titration Of Acid Base Experiment at Joanne Rivera blog Titration To Determine The Concentration Of An Acid Suppose that a titration is performed and \(20.70 \: Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A titration can be performed with. Titrating strong acids and strong bases. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. Performing. Titration To Determine The Concentration Of An Acid.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration To Determine The Concentration Of An Acid \ce{naoh}\) is required to reach the end point when titrated. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. In a titration experiment, a solution. Here is how to perform the. Titration To Determine The Concentration Of An Acid.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration To Determine The Concentration Of An Acid A titration can be performed with. Titrating strong acids and strong bases. Titrating a weak acid with a strong base. It is based on the neutralization reaction, where. Here is how to perform the calculation. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Performing chemical reactions quantitatively to determine. Titration To Determine The Concentration Of An Acid.
From joioqtatt.blob.core.windows.net
Types Of Titration Reactions at Alex Ortiz blog Titration To Determine The Concentration Of An Acid When we want to determine the concentration of an acid or a base, we can perform a titration experiment. Suppose that a titration is performed and \(20.70 \: Here is how to perform the calculation. It is based on the neutralization reaction, where. A chemical reaction is set up between a known volume of a solution of unknown concentration and. Titration To Determine The Concentration Of An Acid.
From mungfali.com
Acid Base Titration Method Titration To Determine The Concentration Of An Acid Here is how to perform the calculation. Titrating a weak acid with a strong base. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. Titrating strong acids and strong bases. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. A. Titration To Determine The Concentration Of An Acid.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration To Determine The Concentration Of An Acid Here is how to perform the calculation. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. It is based on the neutralization reaction, where. A titration can be performed with. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution. Titration To Determine The Concentration Of An Acid.
From www.youtube.com
Calculating the concentration of an acid by titration YouTube Titration To Determine The Concentration Of An Acid Suppose that a titration is performed and \(20.70 \: In a titration experiment, a solution. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Titrating a weak acid with a strong base. When we want to determine the concentration of an acid. Titration To Determine The Concentration Of An Acid.
From studylib.net
AcidBase Titration Curve Titration To Determine The Concentration Of An Acid Titrating strong acids and strong bases. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. It is based on the neutralization reaction, where. A chemical reaction is set up between a known volume of. Titration To Determine The Concentration Of An Acid.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration To Determine The Concentration Of An Acid Titrating a weak acid with a strong base. Here is how to perform the calculation. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. In a titration experiment, a solution. Titrating strong acids and strong bases. \ce{naoh}\) is required to reach the. Titration To Determine The Concentration Of An Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration To Determine The Concentration Of An Acid Here is how to perform the calculation. A titration can be performed with. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titrating strong acids and strong bases. It is based on the neutralization reaction, where. In a titration experiment, a solution. \ce{naoh}\) is required to reach the end point. Titration To Determine The Concentration Of An Acid.
From ceavsgvd.blob.core.windows.net
How To Calculate Acid Concentration From Titration at Susan Carrier blog Titration To Determine The Concentration Of An Acid \ce{naoh}\) is required to reach the end point when titrated. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. Titrating strong acids and strong bases. Suppose that a titration is performed and \(20.70 \: Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration.. Titration To Determine The Concentration Of An Acid.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration To Determine The Concentration Of An Acid It is based on the neutralization reaction, where. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. In a titration experiment, a solution. When we want to determine the concentration of an acid or a base, we can perform a titration experiment.. Titration To Determine The Concentration Of An Acid.
From www.youtube.com
Finding concentration of acid or base given amount needed to titrate Titration To Determine The Concentration Of An Acid Suppose that a titration is performed and \(20.70 \: Titrating a weak acid with a strong base. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. \ce{naoh}\) is required to reach. Titration To Determine The Concentration Of An Acid.
From www.chegg.com
Solved Determination of unknown concentration of an acid or Titration To Determine The Concentration Of An Acid A titration can be performed with. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. Here is how to perform the calculation. \ce{naoh}\) is required to. Titration To Determine The Concentration Of An Acid.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration To Determine The Concentration Of An Acid Titrating strong acids and strong bases. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. In a titration experiment, a solution. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Here is how to perform the calculation. \ce{naoh}\) is required to reach the. Titration To Determine The Concentration Of An Acid.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration To Determine The Concentration Of An Acid Titrating a weak acid with a strong base. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. A titration can be performed with. Performing chemical reactions quantitatively to determine the exact. Titration To Determine The Concentration Of An Acid.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Titration To Determine The Concentration Of An Acid A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titrating strong acids and strong bases. \ce{naoh}\) is required to reach the end point. Titration To Determine The Concentration Of An Acid.
From mungfali.com
Acid Base Titration Procedure Titration To Determine The Concentration Of An Acid Here is how to perform the calculation. Titrating a weak acid with a strong base. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. It is based on the neutralization reaction, where. Titrating strong acids and strong bases. In a titration experiment, a solution. Suppose that a titration is performed and \(20.70 \:. Titration To Determine The Concentration Of An Acid.
From www.compoundchem.com
A Guide to Acids, Acid Strength, and Concentration Compound Interest Titration To Determine The Concentration Of An Acid It is based on the neutralization reaction, where. \ce{naoh}\) is required to reach the end point when titrated. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. In a titration experiment, a solution. Here. Titration To Determine The Concentration Of An Acid.
From www.sliderbase.com
Titration Titration To Determine The Concentration Of An Acid A titration can be performed with. Titrating a weak acid with a strong base. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titration. In a titration experiment, a solution. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with. Titration To Determine The Concentration Of An Acid.
From www.youtube.com
Titration of an unknown acid with a standardized Sodium Hydroxide Titration To Determine The Concentration Of An Acid Here is how to perform the calculation. \ce{naoh}\) is required to reach the end point when titrated. Titrating strong acids and strong bases. Suppose that a titration is performed and \(20.70 \: In a titration experiment, a solution. It is based on the neutralization reaction, where. When we want to determine the concentration of an acid or a base, we. Titration To Determine The Concentration Of An Acid.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration To Determine The Concentration Of An Acid Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Titrating a weak acid with a strong base. A titration can be performed with. Titrating strong acids and strong bases. Here is how to perform the calculation. Suppose that a titration is performed and \(20.70 \: Performing chemical reactions quantitatively to. Titration To Determine The Concentration Of An Acid.
From fity.club
Concentration Formula Titration To Determine The Concentration Of An Acid A titration can be performed with. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. Titrating strong acids and strong bases. Titrating a weak acid with a strong base. When we want to determine the concentration of an acid or a base,. Titration To Determine The Concentration Of An Acid.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube Titration To Determine The Concentration Of An Acid It is based on the neutralization reaction, where. A titration can be performed with. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. \ce{naoh}\) is required to reach the end point when titrated. Titrating a weak acid with a strong base. In a titration experiment, a solution. Titrating strong acids. Titration To Determine The Concentration Of An Acid.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Titration To Determine The Concentration Of An Acid It is based on the neutralization reaction, where. \ce{naoh}\) is required to reach the end point when titrated. When we want to determine the concentration of an acid or a base, we can perform a titration experiment. Titrating strong acids and strong bases. In a titration experiment, a solution. Titration is a procedure used in chemistry in order to determine. Titration To Determine The Concentration Of An Acid.