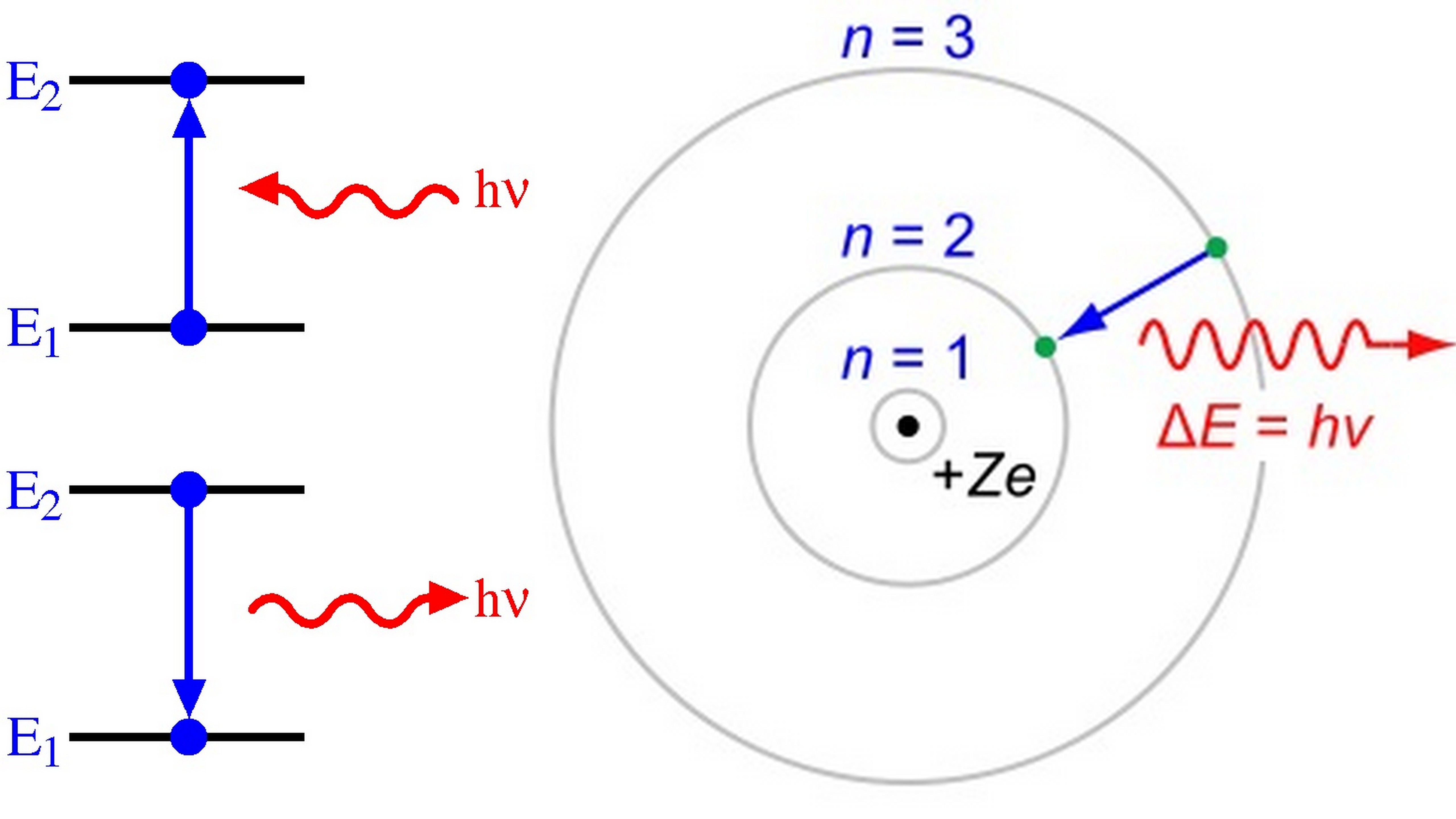
Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain . When the electron absorbs a photon,. The energy difference between the energy levels of. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. What happens if the photon has. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough energy to ionize the atom. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6 ev). Yes, the electron in the ground state of hydrogen can absorb a photon of energy 13.6ev or greater.
from www.naturphilosophie.co.uk
What happens if the photon has. So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough energy to ionize the atom. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6 ev). The energy difference between the energy levels of. Yes, the electron in the ground state of hydrogen can absorb a photon of energy 13.6ev or greater. When the electron absorbs a photon,. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy.
At the Heart of the Hydrogen Atom... NaturPhilosophie
Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. What happens if the photon has. Yes, the electron in the ground state of hydrogen can absorb a photon of energy 13.6ev or greater. An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. The energy difference between the energy levels of. When the electron absorbs a photon,. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6 ev). A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough energy to ionize the atom.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough energy to ionize the atom. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. When the electron absorbs a photon,. An electron in the ground state of a hydrogen atom can. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. What happens if the photon has. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6 ev). So, if a photon with. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.numerade.com
SOLVED 26. Can a hydrogen atom absorb a photon whose energy exceeds Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. So, if a photon with energy greater than. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.numerade.com
SOLVED A hydrogen atom initially in the n = 2 level emits a photon and Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain What happens if the photon has. The energy difference between the energy levels of. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough energy to ionize the atom. 1 the energy of the photon (14. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.toppr.com
A hydrogen atom initially in its ground state absorbs a photon and is Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain The energy difference between the energy levels of. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6 ev). A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. What happens if the photon has. So, if a. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From studylib.net
Absorption / Emission of Photons and Conservation of Energy Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. When the electron absorbs a photon,. Yes, the electron in the ground state of hydrogen can absorb a photon of energy 13.6ev or greater. So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom,. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.chegg.com
Solved When a hydrogen atom absorbs a photon of Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6 ev). The energy difference between the energy levels of. An electron in the ground. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.numerade.com
SOLVED(a) Can a hydrogen atom in the ground state absorb an Halpha Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. When the electron absorbs a photon,. So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom,. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.toppr.com
The ionization energy of hydrogen is 13.6eV. The energy of the photon Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough energy to ionize the atom. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. When the electron. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.toppr.com
A hydrogen atom (ionization energy 13.6eV ) jumps from third excited Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. When the electron absorbs a photon,. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From chem.libretexts.org
Chapter 2.3 Atomic Spectra and Models of the Atom Chemistry LibreTexts Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain The energy difference between the energy levels of. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.youtube.com
Quantized Energy and Photons Chemistry Tutorial YouTube Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain The energy difference between the energy levels of. So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough energy to ionize the atom. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 =. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.numerade.com
SOLVEDCan a hydrogen atom absorb a photon whose energy is greater than Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. What happens if the photon has. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6 ev). When the electron absorbs a photon,. The. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From byjus.com
the energy of hydrogen atom in ground state is 13.6eV.its energy Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain When the electron absorbs a photon,. The energy difference between the energy levels of. So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough energy to ionize the atom. An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. Yes,. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.youtube.com
What is the wavelength of light emitted when the electron in a hydrogen Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. So, if a photon with energy greater than. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.naturphilosophie.co.uk
At the Heart of the Hydrogen Atom... NaturPhilosophie Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. What happens if the photon has. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. Yes, the electron in the ground state of hydrogen can absorb a photon of energy 13.6ev. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. What happens if the photon has. Yes, the electron in the ground state of hydrogen can absorb a photon of energy 13.6ev or greater. An electron in the. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain Yes, the electron in the ground state of hydrogen can absorb a photon of energy 13.6ev or greater. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. The energy difference between the energy levels of. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. An electron. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.chegg.com
Solved 7. A hydrogen atom absorbs a photon of UV light, and Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain Yes, the electron in the ground state of hydrogen can absorb a photon of energy 13.6ev or greater. The energy difference between the energy levels of. When the electron absorbs a photon,. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From slideplayer.com
PHYS 3313 Section 001 Lecture 13 ppt download Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. What happens if the photon has. When the electron absorbs a photon,. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From grapirewocircuitfix.z14.web.core.windows.net
Energy Diagram For Hydrogen Atom Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6 ev). A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. An electron in the ground state of a hydrogen atom can absorb a photon of energy greater. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.youtube.com
How To Calculate The Energy of a Photon Given Frequency & Wavelength in Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough energy to ionize the atom. When the electron absorbs a photon,. An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. 1 the energy of the photon (14 ev) is. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From askfilo.com
The ground state energy of hydrogen atom is −13.6eV. The photon emitted d.. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain When the electron absorbs a photon,. What happens if the photon has. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. So, if a photon with energy greater than 13.6 ev is absorbed by a. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From byjus.com
when an electron in hydrogen atom absorb a photon of energy 12.75eV its Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain The energy difference between the energy levels of. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6 ev). A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. Yes, the electron in the ground state of hydrogen. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.toppr.com
The ionisation potential of hydrogen atom is 13.6 eV. An electron in Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain When the electron absorbs a photon,. Yes, the electron in the ground state of hydrogen can absorb a photon of energy 13.6ev or greater. The energy difference between the energy levels of. What happens if the photon has. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 =. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.chegg.com
Solved 11. A hydrogen atom absorbs a photon of UV light, and Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough energy to ionize the atom. 1 the energy of the photon (14 ev) is greater than the ground state energy of the. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.toppr.com
The ionisation potential of hydrogen atom is 13.6 eV. An electron in Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain When the electron absorbs a photon,. What happens if the photon has. Yes, the electron in the ground state of hydrogen can absorb a photon of energy 13.6ev or greater. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6 ev). A. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.toppr.com
A hydrogen atom, initially in the ground state is excited by absorbing Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6 ev). The energy difference between the energy levels of. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. When the electron absorbs a. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.doubtnut.com
Ionization potential of hydrogen atom is 13.6 eV. Hydrogen atoms in the Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. When the electron absorbs a photon,. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.numerade.com
SOLVED The ground state hydrogen atom absorbs a photon of light having Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.youtube.com
Assertion A hydrogen atom can absorb a photon whose energy is greater Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain When the electron absorbs a photon,. What happens if the photon has. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its binding energy. 1 the energy of the photon (14 ev) is greater than the ground state energy of. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From byjus.com
Ground state energy of an H atom is 13.6eV. The energy needed to Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain What happens if the photon has. A hydrogen atom cannot absorb a photon with an energy greater than 13.6 ev. When the electron absorbs a photon,. Yes, the electron in the ground state of hydrogen can absorb a photon of energy 13.6ev or greater. A hydrogen atom cannot absorb a photon whose energy is greater than 13.6 e v, its. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.animalia-life.club
Electron Energy Levels Hydrogen Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain When the electron absorbs a photon,. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6 e_{0}=13.6 e 0 = 13.6 ev). So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough energy to ionize the atom.. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From askfilo.com
A photon of energy 12.09eV is completely absorbed by a hydrogen atom init.. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough energy to ionize the atom. An electron in the ground state of a hydrogen atom can absorb a photon of energy greater than 13.6 ev. When the electron absorbs a photon,. The energy difference between the energy levels of. A. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.
From www.slideshare.net
Photon and energy levels Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain When the electron absorbs a photon,. What happens if the photon has. So, if a photon with energy greater than 13.6 ev is absorbed by a hydrogen atom, it will have enough energy to ionize the atom. 1 the energy of the photon (14 ev) is greater than the ground state energy of the hydrogen atom (e 0 = 13.6. Can Hydrogen Atom Absorb A Photon Of Energy Greater Than 13 6Ev Explain.