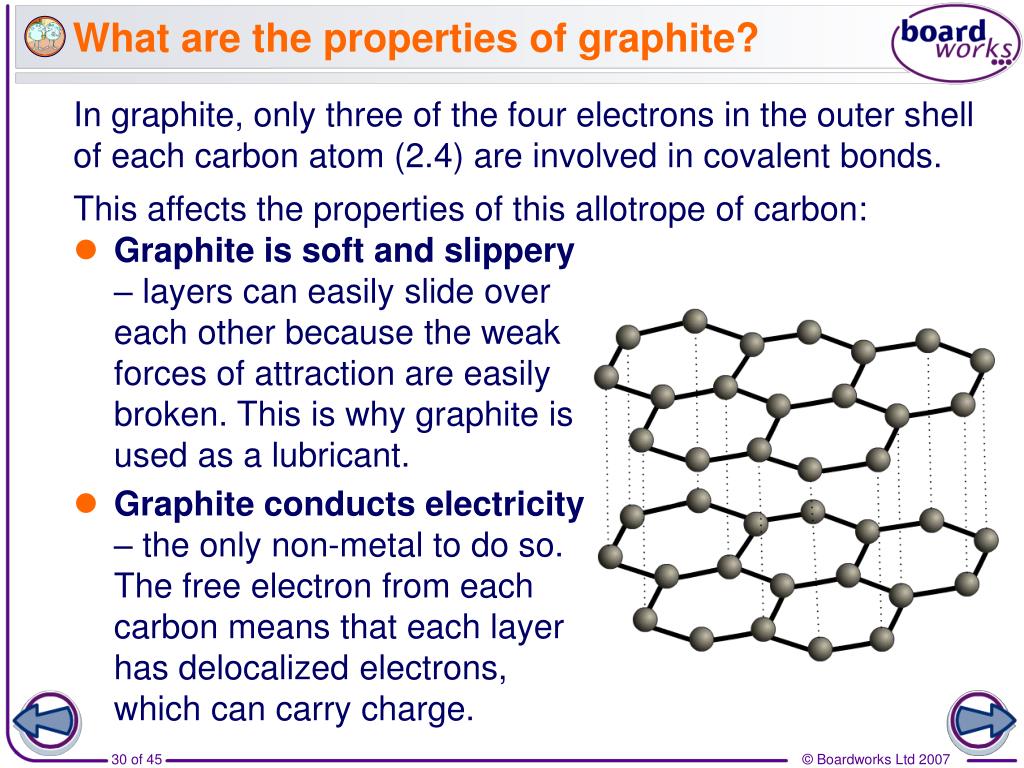
Why Is A Graphite Considered An Element . Graphite is a good conductor of heat and. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. It is a soft, slippery, greyish black substance. Carbon is a nonmetallic element that exists in. Graphite is an allotrope of carbon, which means it is a. Carbon and graphite are both forms of the element carbon, but they have distinct properties and uses. It has a metallic lustre and is opaque to light. Graphite is considered an element because it consists of pure carbon. Graphite is a mineral of extremes. In graphite, each carbon atom is connected to three other carbon atoms. Yes, get the answer no, go search my questions. So let’s dive right into it. Explain why is graphite considered an element? It is a native element mineral found in metamorphic and igneous rocks. It is extremely soft, cleaves with very light pressure, and has a very.
from www.slideserve.com
Graphite is considered an element because it consists of pure carbon. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. In graphite, each carbon atom is connected to three other carbon atoms. It is a soft, slippery, greyish black substance. Carbon is a nonmetallic element that exists in. It is extremely soft, cleaves with very light pressure, and has a very. Graphite is a good conductor of heat and. Carbon and graphite are both forms of the element carbon, but they have distinct properties and uses. Explain why is graphite considered an element? It has a metallic lustre and is opaque to light.
PPT Why do atoms form bonds? PowerPoint Presentation, free download
Why Is A Graphite Considered An Element Graphite is considered an element because it consists of pure carbon. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. In graphite, each carbon atom is connected to three other carbon atoms. Graphite is considered an element because it consists of pure carbon. Yes, get the answer no, go search my questions. Carbon is a nonmetallic element that exists in. It is a soft, slippery, greyish black substance. Graphite is a mineral of extremes. It is extremely soft, cleaves with very light pressure, and has a very. Graphite is an allotrope of carbon, which means it is a. So let’s dive right into it. Carbon and graphite are both forms of the element carbon, but they have distinct properties and uses. It is a native element mineral found in metamorphic and igneous rocks. Explain why is graphite considered an element? It has a metallic lustre and is opaque to light. Graphite is a good conductor of heat and.
From mineralseducationcoalition.org
Graphite Minerals Education Coalition Why Is A Graphite Considered An Element Graphite is a mineral of extremes. Carbon and graphite are both forms of the element carbon, but they have distinct properties and uses. Graphite is considered an element because it consists of pure carbon. It is a soft, slippery, greyish black substance. Graphite is an allotrope of carbon, which means it is a. It is extremely soft, cleaves with very. Why Is A Graphite Considered An Element.
From www.slideserve.com
PPT Why are elements and compounds classified as pure substances Why Is A Graphite Considered An Element It has a metallic lustre and is opaque to light. Graphite is an allotrope of carbon, which means it is a. Explain why is graphite considered an element? Graphite is a mineral of extremes. Yes, get the answer no, go search my questions. It is extremely soft, cleaves with very light pressure, and has a very. Graphite is very soft. Why Is A Graphite Considered An Element.
From physicsopenlab.org
Graphite Structure PhysicsOpenLab Why Is A Graphite Considered An Element It is a native element mineral found in metamorphic and igneous rocks. Graphite is a good conductor of heat and. Graphite is considered an element because it consists of pure carbon. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. It is extremely soft, cleaves with. Why Is A Graphite Considered An Element.
From socratic.org
What are diamond and graphite in relation to carbon? Socratic Why Is A Graphite Considered An Element It is a soft, slippery, greyish black substance. So let’s dive right into it. Graphite is a good conductor of heat and. Graphite is a mineral of extremes. In graphite, each carbon atom is connected to three other carbon atoms. Explain why is graphite considered an element? Graphite is considered an element because it consists of pure carbon. It has. Why Is A Graphite Considered An Element.
From vietkidsiq.edu.vn
Update more than 119 draw the structure of diamond super hot Why Is A Graphite Considered An Element It is a soft, slippery, greyish black substance. It has a metallic lustre and is opaque to light. Carbon and graphite are both forms of the element carbon, but they have distinct properties and uses. Graphite is an allotrope of carbon, which means it is a. Graphite is considered an element because it consists of pure carbon. So let’s dive. Why Is A Graphite Considered An Element.
From socratic.org
What is the chemical formula of graphite? Socratic Why Is A Graphite Considered An Element Graphite is considered an element because it consists of pure carbon. Graphite is a mineral of extremes. It is a soft, slippery, greyish black substance. Carbon is a nonmetallic element that exists in. It has a metallic lustre and is opaque to light. Graphite is a good conductor of heat and. Carbon and graphite are both forms of the element. Why Is A Graphite Considered An Element.
From www.ecograf.com.au
» Graphite EcoGraf Why Is A Graphite Considered An Element It is a native element mineral found in metamorphic and igneous rocks. Graphite is a good conductor of heat and. In graphite, each carbon atom is connected to three other carbon atoms. Yes, get the answer no, go search my questions. So let’s dive right into it. Graphite is considered an element because it consists of pure carbon. Graphite is. Why Is A Graphite Considered An Element.
From www.alamy.com
Graphite is a native element mineral composed by carbon. Sample Stock Why Is A Graphite Considered An Element Yes, get the answer no, go search my questions. Explain why is graphite considered an element? Graphite is a mineral of extremes. It has a metallic lustre and is opaque to light. So let’s dive right into it. Carbon is a nonmetallic element that exists in. In graphite, each carbon atom is connected to three other carbon atoms. Graphite is. Why Is A Graphite Considered An Element.
From igraphite.pl
Graphite Innovation graphite in large and small wholesalers 25 Why Is A Graphite Considered An Element Graphite is a good conductor of heat and. Graphite is a mineral of extremes. Yes, get the answer no, go search my questions. Carbon and graphite are both forms of the element carbon, but they have distinct properties and uses. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms. Why Is A Graphite Considered An Element.
From minhkhuetravel.com
Why Is Diamond Incredibly Hard In Chemistry? Why Is A Graphite Considered An Element So let’s dive right into it. Graphite is an allotrope of carbon, which means it is a. Explain why is graphite considered an element? Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. It has a metallic lustre and is opaque to light. In graphite, each. Why Is A Graphite Considered An Element.
From mwi-inc.com
structure of graphite MWI, Inc. Why Is A Graphite Considered An Element Carbon and graphite are both forms of the element carbon, but they have distinct properties and uses. Explain why is graphite considered an element? In graphite, each carbon atom is connected to three other carbon atoms. Carbon is a nonmetallic element that exists in. So let’s dive right into it. Graphite is very soft because the individual layers of carbon. Why Is A Graphite Considered An Element.
From byjus.com
Describe the structure of Graphite. Why Is A Graphite Considered An Element Graphite is a mineral of extremes. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Yes, get the answer no, go search my questions. It has a metallic lustre and is opaque to light. Graphite is considered an element because it consists of pure carbon. Graphite. Why Is A Graphite Considered An Element.
From www.researchgate.net
Graphitic layers distance? ResearchGate Why Is A Graphite Considered An Element Carbon and graphite are both forms of the element carbon, but they have distinct properties and uses. Yes, get the answer no, go search my questions. Graphite is an allotrope of carbon, which means it is a. It has a metallic lustre and is opaque to light. In graphite, each carbon atom is connected to three other carbon atoms. It. Why Is A Graphite Considered An Element.
From askanydifference.com
Diamond vs Graphite Difference and Comparison Why Is A Graphite Considered An Element So let’s dive right into it. Graphite is a mineral of extremes. Graphite is considered an element because it consists of pure carbon. It is a soft, slippery, greyish black substance. Carbon is a nonmetallic element that exists in. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within. Why Is A Graphite Considered An Element.
From energy.virginia.gov
Virginia Energy Geology and Mineral Resources Graphite Why Is A Graphite Considered An Element In graphite, each carbon atom is connected to three other carbon atoms. Carbon is a nonmetallic element that exists in. Carbon and graphite are both forms of the element carbon, but they have distinct properties and uses. Graphite is an allotrope of carbon, which means it is a. Graphite is a good conductor of heat and. Graphite is very soft. Why Is A Graphite Considered An Element.
From www.alamy.com
Carbon graphite and diamond hires stock photography and images Alamy Why Is A Graphite Considered An Element Carbon is a nonmetallic element that exists in. It is extremely soft, cleaves with very light pressure, and has a very. Graphite is a mineral of extremes. Explain why is graphite considered an element? It is a native element mineral found in metamorphic and igneous rocks. It has a metallic lustre and is opaque to light. So let’s dive right. Why Is A Graphite Considered An Element.
From www.alamy.com
Graphite layers, threedimensional schematic diagram. Crystalline form Why Is A Graphite Considered An Element Graphite is a mineral of extremes. It has a metallic lustre and is opaque to light. Carbon and graphite are both forms of the element carbon, but they have distinct properties and uses. Explain why is graphite considered an element? So let’s dive right into it. Graphite is very soft because the individual layers of carbon atoms are not as. Why Is A Graphite Considered An Element.
From www.alamy.com
Graphite carbon atom Cut Out Stock Images & Pictures Alamy Why Is A Graphite Considered An Element Yes, get the answer no, go search my questions. Graphite is an allotrope of carbon, which means it is a. Graphite is a mineral of extremes. Graphite is a good conductor of heat and. Explain why is graphite considered an element? It is extremely soft, cleaves with very light pressure, and has a very. Graphite is very soft because the. Why Is A Graphite Considered An Element.
From mineralsforsale.rayw.co.uk
graphite a form of pure carbon Rocks and Mineral Specimens for Sale Why Is A Graphite Considered An Element Graphite is an allotrope of carbon, which means it is a. So let’s dive right into it. Yes, get the answer no, go search my questions. It has a metallic lustre and is opaque to light. It is a soft, slippery, greyish black substance. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound. Why Is A Graphite Considered An Element.
From tirupatigraphite.com
Flake Graphite Experts From India Why Is A Graphite Considered An Element Carbon and graphite are both forms of the element carbon, but they have distinct properties and uses. So let’s dive right into it. It is a native element mineral found in metamorphic and igneous rocks. Explain why is graphite considered an element? Graphite is a good conductor of heat and. Graphite is an allotrope of carbon, which means it is. Why Is A Graphite Considered An Element.
From energyeducation.ca
Graphite Energy Education Why Is A Graphite Considered An Element In graphite, each carbon atom is connected to three other carbon atoms. It has a metallic lustre and is opaque to light. Carbon is a nonmetallic element that exists in. Graphite is a mineral of extremes. Graphite is an allotrope of carbon, which means it is a. Graphite is a good conductor of heat and. So let’s dive right into. Why Is A Graphite Considered An Element.
From www.slideserve.com
PPT Covalent Bonds PowerPoint Presentation, free download ID2654690 Why Is A Graphite Considered An Element It is extremely soft, cleaves with very light pressure, and has a very. It is a soft, slippery, greyish black substance. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Graphite is considered an element because it consists of pure carbon. It has a metallic lustre. Why Is A Graphite Considered An Element.
From researchfeatures.com
Carbyne A simple yet strong chain of carbon atoms Why Is A Graphite Considered An Element Graphite is a good conductor of heat and. It is a native element mineral found in metamorphic and igneous rocks. Yes, get the answer no, go search my questions. Graphite is a mineral of extremes. In graphite, each carbon atom is connected to three other carbon atoms. It has a metallic lustre and is opaque to light. It is extremely. Why Is A Graphite Considered An Element.
From byjus.com
Graphite has a shiny appearance so why it is considered as a non metal? Why Is A Graphite Considered An Element Graphite is an allotrope of carbon, which means it is a. Explain why is graphite considered an element? It is extremely soft, cleaves with very light pressure, and has a very. Yes, get the answer no, go search my questions. Graphite is a good conductor of heat and. It is a soft, slippery, greyish black substance. So let’s dive right. Why Is A Graphite Considered An Element.
From grapheneleaderscanada.com
Graphite and Graphene Graphene Leaders Canada Why Is A Graphite Considered An Element Explain why is graphite considered an element? Graphite is considered an element because it consists of pure carbon. It is a soft, slippery, greyish black substance. Graphite is a mineral of extremes. So let’s dive right into it. Graphite is an allotrope of carbon, which means it is a. Yes, get the answer no, go search my questions. Carbon is. Why Is A Graphite Considered An Element.
From byjus.com
Distinguish between graphite and diamond on the basis of their structures. Why Is A Graphite Considered An Element It is extremely soft, cleaves with very light pressure, and has a very. Graphite is an allotrope of carbon, which means it is a. Graphite is a mineral of extremes. Yes, get the answer no, go search my questions. Explain why is graphite considered an element? Graphite is considered an element because it consists of pure carbon. Graphite is a. Why Is A Graphite Considered An Element.
From www.slideserve.com
PPT Why do atoms form bonds? PowerPoint Presentation, free download Why Is A Graphite Considered An Element It is a native element mineral found in metamorphic and igneous rocks. It is a soft, slippery, greyish black substance. It is extremely soft, cleaves with very light pressure, and has a very. It has a metallic lustre and is opaque to light. Graphite is a mineral of extremes. Graphite is an allotrope of carbon, which means it is a.. Why Is A Graphite Considered An Element.
From k-mine.com
Flake Graphite An Integral Element in Fulfilling Global Energy Needs Why Is A Graphite Considered An Element Yes, get the answer no, go search my questions. It is a native element mineral found in metamorphic and igneous rocks. Carbon is a nonmetallic element that exists in. In graphite, each carbon atom is connected to three other carbon atoms. Explain why is graphite considered an element? It has a metallic lustre and is opaque to light. Graphite is. Why Is A Graphite Considered An Element.
From www.askiitians.com
Classification of Crystalline Solids Study Material for IIT JEE Why Is A Graphite Considered An Element So let’s dive right into it. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Carbon is a nonmetallic element that exists in. In graphite, each carbon atom is connected to three other carbon atoms. Carbon and graphite are both forms of the element carbon, but. Why Is A Graphite Considered An Element.
From zh.wikipedia.org
FileDiamond and graphite.jpg 维基百科,自由的百科全书 Why Is A Graphite Considered An Element Graphite is an allotrope of carbon, which means it is a. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Graphite is a mineral of extremes. In graphite, each carbon atom is connected to three other carbon atoms. Graphite is a good conductor of heat and.. Why Is A Graphite Considered An Element.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:1.7.4 Giant Covalent Why Is A Graphite Considered An Element Yes, get the answer no, go search my questions. It is a native element mineral found in metamorphic and igneous rocks. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. Graphite is an allotrope of carbon, which means it is a. So let’s dive right into. Why Is A Graphite Considered An Element.
From www.learnatnoon.com
How is graphite different from diamonds? Noon Academy Why Is A Graphite Considered An Element Explain why is graphite considered an element? Carbon and graphite are both forms of the element carbon, but they have distinct properties and uses. So let’s dive right into it. Yes, get the answer no, go search my questions. It is a native element mineral found in metamorphic and igneous rocks. Graphite is considered an element because it consists of. Why Is A Graphite Considered An Element.
From ar.inspiredpencil.com
Graphite Why Is A Graphite Considered An Element Graphite is a good conductor of heat and. Graphite is an allotrope of carbon, which means it is a. Explain why is graphite considered an element? Graphite is considered an element because it consists of pure carbon. So let’s dive right into it. Graphite is a mineral of extremes. Carbon is a nonmetallic element that exists in. Carbon and graphite. Why Is A Graphite Considered An Element.
From www.slideshare.net
Graphite Why Is A Graphite Considered An Element Graphite is an allotrope of carbon, which means it is a. Graphite is a good conductor of heat and. Carbon and graphite are both forms of the element carbon, but they have distinct properties and uses. It has a metallic lustre and is opaque to light. Explain why is graphite considered an element? It is a native element mineral found. Why Is A Graphite Considered An Element.
From www.savemyexams.com
Structure & Physical Properties OCR A Level Chemistry Revision Notes 2017 Why Is A Graphite Considered An Element Explain why is graphite considered an element? Graphite is an allotrope of carbon, which means it is a. Yes, get the answer no, go search my questions. Graphite is a good conductor of heat and. Graphite is very soft because the individual layers of carbon atoms are not as tightly bound together as the atoms within the layer. It is. Why Is A Graphite Considered An Element.