If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air . The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap bubble to reduce its size. Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. The correct option is a the pressure decreases. As the time passes pressure of the gas. From the first law of thermodynamics, dq = du +dw. Due to compression the temperature of the system increases to a very high value. Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. Sudden processes are adiabatic, so dq = 0. A gas is contained in. Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position.
from www.chegg.com
As the time passes pressure of the gas. A gas is contained in. Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. Sudden processes are adiabatic, so dq = 0. Due to compression the temperature of the system increases to a very high value. Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position. The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap bubble to reduce its size. The correct option is a the pressure decreases. From the first law of thermodynamics, dq = du +dw.
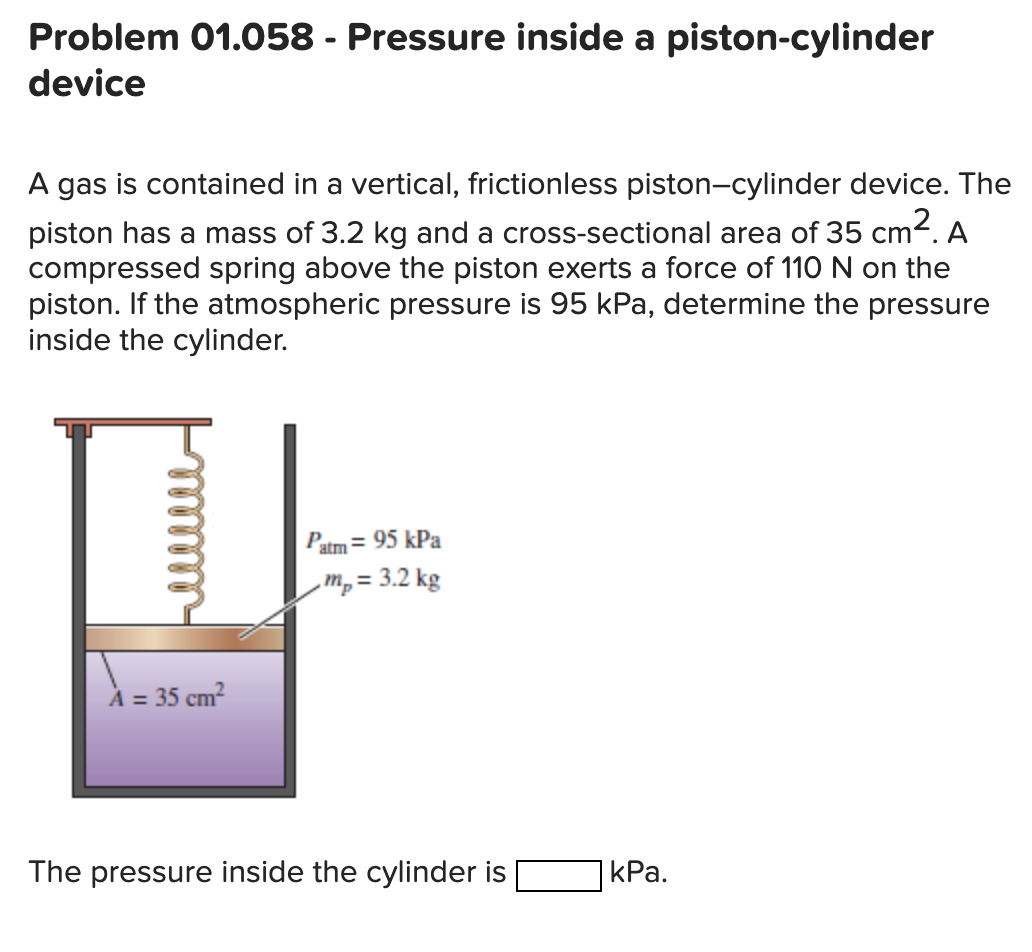
Solved Problem 01.058 Pressure inside a pistoncylinder
If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap bubble to reduce its size. The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap bubble to reduce its size. Sudden processes are adiabatic, so dq = 0. Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. A gas is contained in. Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position. From the first law of thermodynamics, dq = du +dw. The correct option is a the pressure decreases. As the time passes pressure of the gas. Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. Due to compression the temperature of the system increases to a very high value.
From www.solutionspile.com
[Solved] A pistoncylinder arrangement contains air at If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air As the time passes pressure of the gas. Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position. Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. Due to compression the temperature of the system. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.numerade.com
SOLVED Consider a pistoncylinder device as shown in the Figure below If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. As the time passes pressure of the gas. From the first law of thermodynamics, dq = du +dw. The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.doubtnut.com
Air in a cylinder is suddenly compressed by a piston. which is then ma If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Sudden processes are adiabatic, so dq = 0. The correct option is a the pressure decreases. A gas is contained in. From the first law of thermodynamics, dq = du +dw. Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. Air in a cylinder is suddenly compressed by a. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From brainly.com
Air is compressed slowly in a pistoncylinder assembly from an initial If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Sudden processes are adiabatic, so dq = 0. From the first law of thermodynamics, dq = du +dw. As the time passes pressure of the gas. The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap bubble to reduce its size. The correct option is a the pressure. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.doubtnut.com
A monoatomic gas is enclosed in a nonconducting cylinder having a pis If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air A gas is contained in. Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position. Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. As the time passes pressure of the gas. The pressure p. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved Hydrogen gas is compressed in a pistoncylinder If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air A gas is contained in. Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap bubble to reduce its size. Due to compression the temperature of the system increases to. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved Problem 01.058 Pressure inside a pistoncylinder If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. From the first law of thermodynamics, dq = du +dw. The correct option is a the pressure decreases. Sudden processes are adiabatic, so dq = 0. Due to compression the temperature of the system increases to a very high value. A gas. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved 253 A frictionless pistoncylinder device contains If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air As the time passes pressure of the gas. Due to compression the temperature of the system increases to a very high value. The correct option is a the pressure decreases. Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. Determine the specific entropy change and the specific work done during the. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.toppr.com
Air in a cylinder is suddenly compressed by a piston which is then If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Sudden processes are adiabatic, so dq = 0. The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap bubble to reduce its size. The correct option is a the pressure decreases. A gas is contained in. Due to compression the temperature of the system increases to a very. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved Air contained within a pistoncylinder assembly is If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air As the time passes pressure of the gas. The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap bubble to reduce its size. Due to compression the temperature of the system increases to a very high value. From the first law of thermodynamics, dq = du +dw. The. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved Air is contained in a pistoncylinder assembly as If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. The correct option is a the pressure decreases. Due to compression the temperature of the system increases to a very high value. A gas is contained in. The pressure p 2 to which the air should be compressed by slowly pushing the. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved 472 A mass of 15 kg of air in a pistoncylinder If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Sudden processes are adiabatic, so dq = 0. A gas is contained in. Due to compression the temperature of the system increases to a very high value. Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position. Determine the specific entropy change and the specific work. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved Air is held within a frictionless pistoncylinder If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position. Sudden processes are adiabatic, so dq = 0. The correct option is a the pressure decreases. The pressure. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From physicslabs.ccnysites.cuny.edu
Ideal Gas Tutorial If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air As the time passes pressure of the gas. The correct option is a the pressure decreases. Due to compression the temperature of the system increases to a very high value. Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. Air in a cylinder, is suddenly compressed to half of. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.researchgate.net
25 Adiabatic compression of air in a cylinder of a diesel engine If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position. Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. Due to compression the temperature of the system increases to a very high value. As the time passes. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved Air is compressed in a pistoncylinder device from If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position. Due to compression the temperature of the system increases to a very high value. Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. Air in. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved Air is compressed adiabatically in a pistoncylinder If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. As the time passes pressure of the gas. Sudden processes are adiabatic, so dq = 0. Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position.. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.coursehero.com
[Solved] Air in a pistoncylinder assembly undergoes a process from If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Sudden processes are adiabatic, so dq = 0. Due to compression the temperature of the system increases to a very high value. The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap bubble to reduce its size. As the time passes pressure of the gas. A gas is. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved A Piston cylinder is used to isothermally compress If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. From the first law of thermodynamics, dq = du +dw. Due to compression the temperature of the system increases to a very high value. The pressure p 2 to which the air should be compressed by slowly pushing the piston. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.toppr.com
Air in a cylinder is suddenly compressed by a piston, which is then If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air As the time passes pressure of the gas. Sudden processes are adiabatic, so dq = 0. From the first law of thermodynamics, dq = du +dw. Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position. Due to compression the temperature of the system increases to. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.youtube.com
PISTONCYLINDER Work and Constant Pressure Example in 5 Minutes! YouTube If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position. The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap bubble to reduce its size. The correct option is a the pressure decreases. Air. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.numerade.com
SOLVEDA piston/cylinder setup containing air at 100 kPa and 400 K is If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position. The correct option is a the pressure decreases. Sudden processes are adiabatic, so dq = 0. Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. Due to. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.numerade.com
A gas is contained in a vertical, frictionless pistoncylinder device If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air The correct option is a the pressure decreases. Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. From the first law of thermodynamics, dq = du +dw. Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. Air in a cylinder,. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.britannica.com
Compressed air Energy Efficiency, Industrial Uses & Safety Britannica If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air A gas is contained in. The correct option is a the pressure decreases. Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position. Sudden processes are adiabatic, so dq = 0. Air in a cylinder is suddenly compressed by a piston which is then maintained at. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved Air contained within a pistoncylinder assembly is If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. A gas is contained in. As the time passes pressure of the gas. The pressure p 2 to which the air should. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved Problem 3 Air (assume ideal gas) is contained in a If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air The correct option is a the pressure decreases. From the first law of thermodynamics, dq = du +dw. A gas is contained in. The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap bubble to reduce its size. Determine the specific entropy change and the specific work done. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved Air expanding in a pistoncylinder assembly Known If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Due to compression the temperature of the system increases to a very high value. The correct option is a the pressure decreases. Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved A Gas Within A Pistoncylinder Assembly Undergoes If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. Sudden processes are adiabatic, so dq = 0. The pressure p 2 to which the air should be compressed by slowly pushing. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.youtube.com
Air in a cylinder is suddenly compressed by a piston, which is then If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Due to compression the temperature of the system increases to a very high value. From the first law of thermodynamics, dq = du +dw. A gas is contained in. The correct option is a the pressure decreases. The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap bubble. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved A frictionless pistoncylinder device contains air If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Due to compression the temperature of the system increases to a very high value. Sudden processes are adiabatic, so dq = 0. From the first law of thermodynamics, dq = du +dw. Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. Air in a cylinder, is suddenly compressed to half of. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.numerade.com
a gas is contained in a vertical frictionless piston cylinder device If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. Due to compression the temperature of the system increases to a very high value. From the first law of thermodynamics, dq = du +dw. The pressure p 2 to which the air should be compressed by slowly pushing the piston into the. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved A gas is compressed by a piston in a cylinder, as If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. A gas is contained in. Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved Q4. A compressed air cylinder is connected to the If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Due to compression the temperature of the system increases to a very high value. Air in a cylinder is suddenly compressed by a piston which is then maintained at the same position. From the first law of thermodynamics, dq = du +dw. A gas is contained in. Sudden processes are adiabatic, so dq = 0. The pressure p 2 to. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.chegg.com
Solved 4. As shown, a gas contained within a pistoncylinder If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air The correct option is a the pressure decreases. Sudden processes are adiabatic, so dq = 0. From the first law of thermodynamics, dq = du +dw. The pressure p 2 to which the air should be compressed by slowly pushing the piston into the cylinder for the soap bubble to reduce its size. Determine the specific entropy change and the. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.
From www.youtube.com
A pistoncylinder device contains 0.15 kg of air initially at 2 MPa and If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air Air in a cylinder, is suddenly compressed to half of its initial volume by a piston which is then maintained at that new position. Sudden processes are adiabatic, so dq = 0. Determine the specific entropy change and the specific work done during the reversible isothermal compression of air in a piston. Air in a cylinder is suddenly compressed by. If Air In A Cylinder Is Suddenly Compressed By A Piston. What Happens To The Pressure Of Air.