Why Is Phenol Not Soluble In Water . the solubility of phenol in water is governed by the hydroxyl group present. If you try to dissolve more than this, you get two. phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. If you try to dissolve more. Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c 6 h 6) with the help of an iron (iii) chloride (fecl 3) catalyst. The hydroxyl group in phenol is involved in the formation.
from www.numerade.com
b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. If you try to dissolve more than this, you get two. The hydroxyl group in phenol is involved in the formation. Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c 6 h 6) with the help of an iron (iii) chloride (fecl 3) catalyst. phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. the solubility of phenol in water is governed by the hydroxyl group present. If you try to dissolve more. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water.
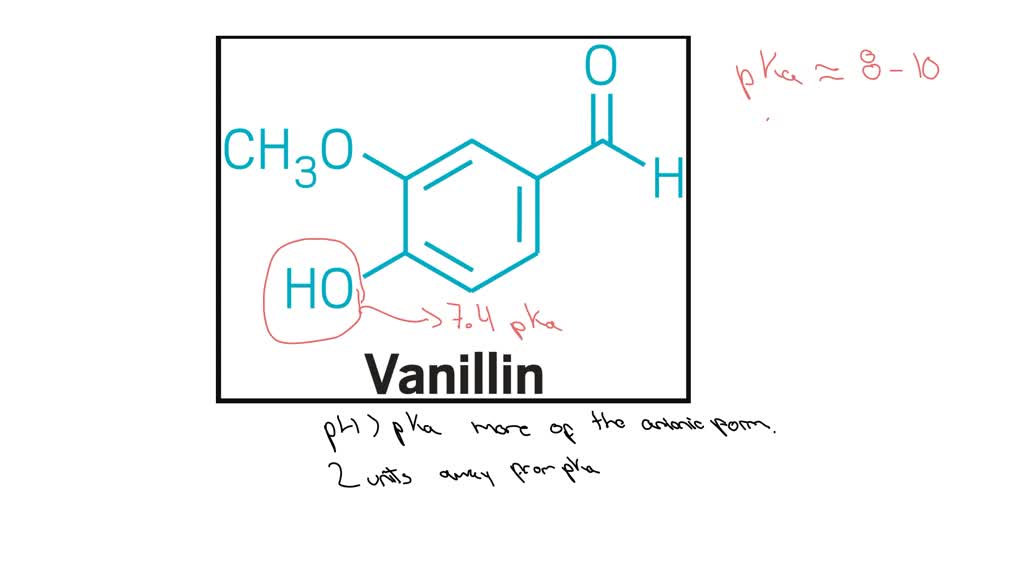
Why does vanillin dissolve in an aqueous NaOH solution, but not in
Why Is Phenol Not Soluble In Water the solubility of phenol in water is governed by the hydroxyl group present. the solubility of phenol in water is governed by the hydroxyl group present. b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. If you try to dissolve more. The hydroxyl group in phenol is involved in the formation. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c 6 h 6) with the help of an iron (iii) chloride (fecl 3) catalyst. If you try to dissolve more than this, you get two.
From www.numerade.com
SOLVED ALCOHOLS, PHENOLS CARBOXYLIC ACIDS Solubility in Water (cross Why Is Phenol Not Soluble In Water If you try to dissolve more. The hydroxyl group in phenol is involved in the formation. Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c 6 h 6) with the help of an iron (iii) chloride. Why Is Phenol Not Soluble In Water.
From physicalpharmacy1424.blogspot.com
Physical Pharmacy Practical Practical 2 Phase Diagram Mutual Why Is Phenol Not Soluble In Water phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. The hydroxyl group in phenol is involved in the formation. If you try to dissolve more. b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. If you try to dissolve more than this, you get two.. Why Is Phenol Not Soluble In Water.
From www.numerade.com
B) DATA FOR SOLUBILITY IN WATER (see sample data table for results of Why Is Phenol Not Soluble In Water If you try to dissolve more. the solubility of phenol in water is governed by the hydroxyl group present. b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. Hydrolysis of chlorobenzene (dow process) dow process. Why Is Phenol Not Soluble In Water.
From slideplayer.com
Unit 6 Alcohols and Ethers ppt download Why Is Phenol Not Soluble In Water If you try to dissolve more than this, you get two. b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. The hydroxyl group in phenol is involved in the formation. Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic. Why Is Phenol Not Soluble In Water.
From netsolwater.com
Why is it necessary to remove phenols from wastewater Why Is Phenol Not Soluble In Water If you try to dissolve more. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c. Why Is Phenol Not Soluble In Water.
From slideplayer.com
Organic Chemistry CHEM ppt download Why Is Phenol Not Soluble In Water b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. If you try to dissolve more. The hydroxyl group in phenol is involved in the formation. Hydrolysis of chlorobenzene (dow. Why Is Phenol Not Soluble In Water.
From www.chegg.com
Solved A Properties of Alcohols and Phenols Table 1. Why Is Phenol Not Soluble In Water The hydroxyl group in phenol is involved in the formation. b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in. Why Is Phenol Not Soluble In Water.
From slideplayer.com
13.1 Alcohols, Phenols, Thiols, and Ethers ppt download Why Is Phenol Not Soluble In Water The hydroxyl group in phenol is involved in the formation. If you try to dissolve more than this, you get two. b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. If you try to dissolve more. phenol dissolves to give a 9.3 percent solution in water, compared with a. Why Is Phenol Not Soluble In Water.
From slideplayer.com
13.1 Alcohols, Phenols, Thiols, and Ethers ppt download Why Is Phenol Not Soluble In Water the solubility of phenol in water is governed by the hydroxyl group present. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. The hydroxyl group in phenol is involved in the formation. If you try to dissolve more. phenol is reasonably soluble in water, much more. Why Is Phenol Not Soluble In Water.
From www.slideserve.com
PPT Alcohols, Phenols, Thiols, and Ethers PowerPoint Presentation Why Is Phenol Not Soluble In Water phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. The hydroxyl group in phenol is involved in the formation. If you try to dissolve more. Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom. Why Is Phenol Not Soluble In Water.
From mutualsolubilitycurve20163b.blogspot.com
Lab Report for Experiment 3b MUTUAL SOLUBILITY CURVE FOR PHENOL AND WATER Why Is Phenol Not Soluble In Water b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. the solubility of phenol in water is governed by the hydroxyl group present. If you try to dissolve more. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water.. Why Is Phenol Not Soluble In Water.
From fyoqykvxc.blob.core.windows.net
Phenol Solubility In Water Temperature at David Hartzell blog Why Is Phenol Not Soluble In Water Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c 6 h 6) with the help of an iron (iii) chloride (fecl 3) catalyst. phenol dissolves to give a 9.3 percent solution in water, compared with. Why Is Phenol Not Soluble In Water.
From www.semanticscholar.org
Table 2 from Solubility of Phenolic Compounds in Pure Water and Why Is Phenol Not Soluble In Water b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. If you try to dissolve more. Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c 6 h. Why Is Phenol Not Soluble In Water.
From slidetodoc.com
Solubility Rules and Precipitation Reactions Chapter 7 Reactions Why Is Phenol Not Soluble In Water the solubility of phenol in water is governed by the hydroxyl group present. b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. If you try to dissolve more. Why Is Phenol Not Soluble In Water.
From exyrxopgj.blob.core.windows.net
What Is Phenol More Soluble In Water Than Toluene at Cassandra Kennedy blog Why Is Phenol Not Soluble In Water b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. If you try to dissolve more than this, you get two. The hydroxyl group in phenol is involved in the formation. the solubility of phenol in water is governed by the hydroxyl group present. phenol dissolves to give a. Why Is Phenol Not Soluble In Water.
From www.scribd.com
Water Phenol PDF Solubility Phase (Matter) Why Is Phenol Not Soluble In Water the solubility of phenol in water is governed by the hydroxyl group present. If you try to dissolve more than this, you get two. Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c 6 h. Why Is Phenol Not Soluble In Water.
From www.numerade.com
SOLVED B) DATA FOR SOLUBILITY IN WATER (see sample data table for Why Is Phenol Not Soluble In Water If you try to dissolve more. phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. If you try to dissolve more than this, you get two. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. b) phenol is more soluble in water. Why Is Phenol Not Soluble In Water.
From www.numerade.com
SOLVED Alcohols and Phenols Experiment Identification of an Unknown Why Is Phenol Not Soluble In Water b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. The hydroxyl group in phenol is involved in the formation. If you try to dissolve more than this, you get two. phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. the solubility of phenol in. Why Is Phenol Not Soluble In Water.
From www.numerade.com
SOLVED Text Physical Properties of Alcohols and Phenols Alcohol pH Why Is Phenol Not Soluble In Water phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. If you try to dissolve more than this, you get two. The hydroxyl group in phenol is involved in the formation. the solubility of phenol in water is governed by the hydroxyl group present. b) phenol is. Why Is Phenol Not Soluble In Water.
From www.numerade.com
SOLVED Phenol is soluble in diethyl ether but not water; however Why Is Phenol Not Soluble In Water Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c 6 h 6) with the help of an iron (iii) chloride (fecl 3) catalyst. phenol dissolves to give a 9.3 percent solution in water, compared with. Why Is Phenol Not Soluble In Water.
From exyrxopgj.blob.core.windows.net
What Is Phenol More Soluble In Water Than Toluene at Cassandra Kennedy blog Why Is Phenol Not Soluble In Water The hydroxyl group in phenol is involved in the formation. If you try to dissolve more. phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. the solubility of phenol in water is governed. Why Is Phenol Not Soluble In Water.
From giojwjxmj.blob.core.windows.net
What Are Phenols In Water at Angela Meekins blog Why Is Phenol Not Soluble In Water Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c 6 h 6) with the help of an iron (iii) chloride (fecl 3) catalyst. the solubility of phenol in water is governed by the hydroxyl group. Why Is Phenol Not Soluble In Water.
From www.bartleby.com
Answered 4.) Phenol Why slightly soluble in… bartleby Why Is Phenol Not Soluble In Water b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. the solubility of phenol in water is governed by the hydroxyl group present. If you try to dissolve more than this, you get two. Hydrolysis of. Why Is Phenol Not Soluble In Water.
From www.youtube.com
2,4dinitro phenol is soluble in NaHCO3 solution but phenol is Why Is Phenol Not Soluble In Water Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c 6 h 6) with the help of an iron (iii) chloride (fecl 3) catalyst. phenol is reasonably soluble in water, much more soluble than benzene and. Why Is Phenol Not Soluble In Water.
From slideplayer.com
Phenols. ppt download Why Is Phenol Not Soluble In Water phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. The hydroxyl group in phenol is involved in the formation. the solubility of phenol in water is governed by the hydroxyl group present. If you try to dissolve more than this, you get two. b) phenol is more soluble in water than cyclohexanol because. Why Is Phenol Not Soluble In Water.
From peterkruwwatson.blogspot.com
Is Phenol Soluble in Water PeterkruwWatson Why Is Phenol Not Soluble In Water b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c 6 h 6) with the help of an. Why Is Phenol Not Soluble In Water.
From www.numerade.com
SOLVED Classification Physical Properties of Alcohols and Phenols Why Is Phenol Not Soluble In Water Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c 6 h 6) with the help of an iron (iii) chloride (fecl 3) catalyst. the solubility of phenol in water is governed by the hydroxyl group. Why Is Phenol Not Soluble In Water.
From slideplayer.com
Alcohols, Ethers and Thiols ppt download Why Is Phenol Not Soluble In Water phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. If you try to dissolve more than this, you get two. The hydroxyl group in phenol is involved in the formation. Hydrolysis of chlorobenzene (dow process) dow. Why Is Phenol Not Soluble In Water.
From fyoqykvxc.blob.core.windows.net
Phenol Solubility In Water Temperature at David Hartzell blog Why Is Phenol Not Soluble In Water phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. the solubility of phenol in water is governed by the hydroxyl group present. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. The hydroxyl group in phenol is involved in the formation. . Why Is Phenol Not Soluble In Water.
From www.youtube.com
Ethanal is soluble in water. Why? YouTube Why Is Phenol Not Soluble In Water phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. b) phenol is more soluble in water than cyclohexanol because of the more polar character of its ring. the solubility of phenol in water is governed by the hydroxyl group present. The hydroxyl group in phenol is involved in the formation. If you try. Why Is Phenol Not Soluble In Water.
From www.numerade.com
SOLVED Phenol is more soluble than toluene in water! WHY? Why Is Phenol Not Soluble In Water If you try to dissolve more than this, you get two. phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. If you try to dissolve more. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. The hydroxyl group in phenol is involved in. Why Is Phenol Not Soluble In Water.
From www.youtube.com
PhenolWater System, Chemistry Lecture Sabaq.pk YouTube Why Is Phenol Not Soluble In Water the solubility of phenol in water is governed by the hydroxyl group present. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. If you try to dissolve more than this, you get two. Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c. Why Is Phenol Not Soluble In Water.
From www.slideserve.com
PPT 5.4.2 Organic nitrogen compounds amines, amides, amino acids and Why Is Phenol Not Soluble In Water If you try to dissolve more. phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. Hydrolysis of chlorobenzene (dow process) dow process starts with the preparation of chlorobenzene (c 6 h 5 cl) from electrophilic aromatic substitution, where chlorine (cl 2) replaces a hydrogen atom in benzene (c 6 h 6) with the help of. Why Is Phenol Not Soluble In Water.
From www.numerade.com
Why does vanillin dissolve in an aqueous NaOH solution, but not in Why Is Phenol Not Soluble In Water the solubility of phenol in water is governed by the hydroxyl group present. The hydroxyl group in phenol is involved in the formation. phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. If. Why Is Phenol Not Soluble In Water.
From www.numerade.com
Both phenol and cyclohexanol are only slightly soluble in water Why Is Phenol Not Soluble In Water phenol is reasonably soluble in water, much more soluble than benzene and methylbenzene. the solubility of phenol in water is governed by the hydroxyl group present. phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. If you try to dissolve more than this, you get two.. Why Is Phenol Not Soluble In Water.