Lab On Solubility . the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. Determine the ratio of anions and cations that create a neutral. collect experimental data and create a solubility curve. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. Explore your finding from this practical into the effect of temperature on solubility. By the end of this lab, students should be able to: Compare the number of ions in solution. Rank the solubility of different salts. To understand how temperature, pressure, and the presence of other solutes affect the solubility of. add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. hot or cold, which water is better for soluble substances? Includes kit list and safety instructions. The solubility of a substance could depend upon the size of the solute pieces, the choice or temperature.
from www.studocu.com
Includes kit list and safety instructions. collect experimental data and create a solubility curve. Rank the solubility of different salts. Determine the ratio of anions and cations that create a neutral. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. To understand how temperature, pressure, and the presence of other solutes affect the solubility of. By the end of this lab, students should be able to: add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. hot or cold, which water is better for soluble substances? Explore your finding from this practical into the effect of temperature on solubility.
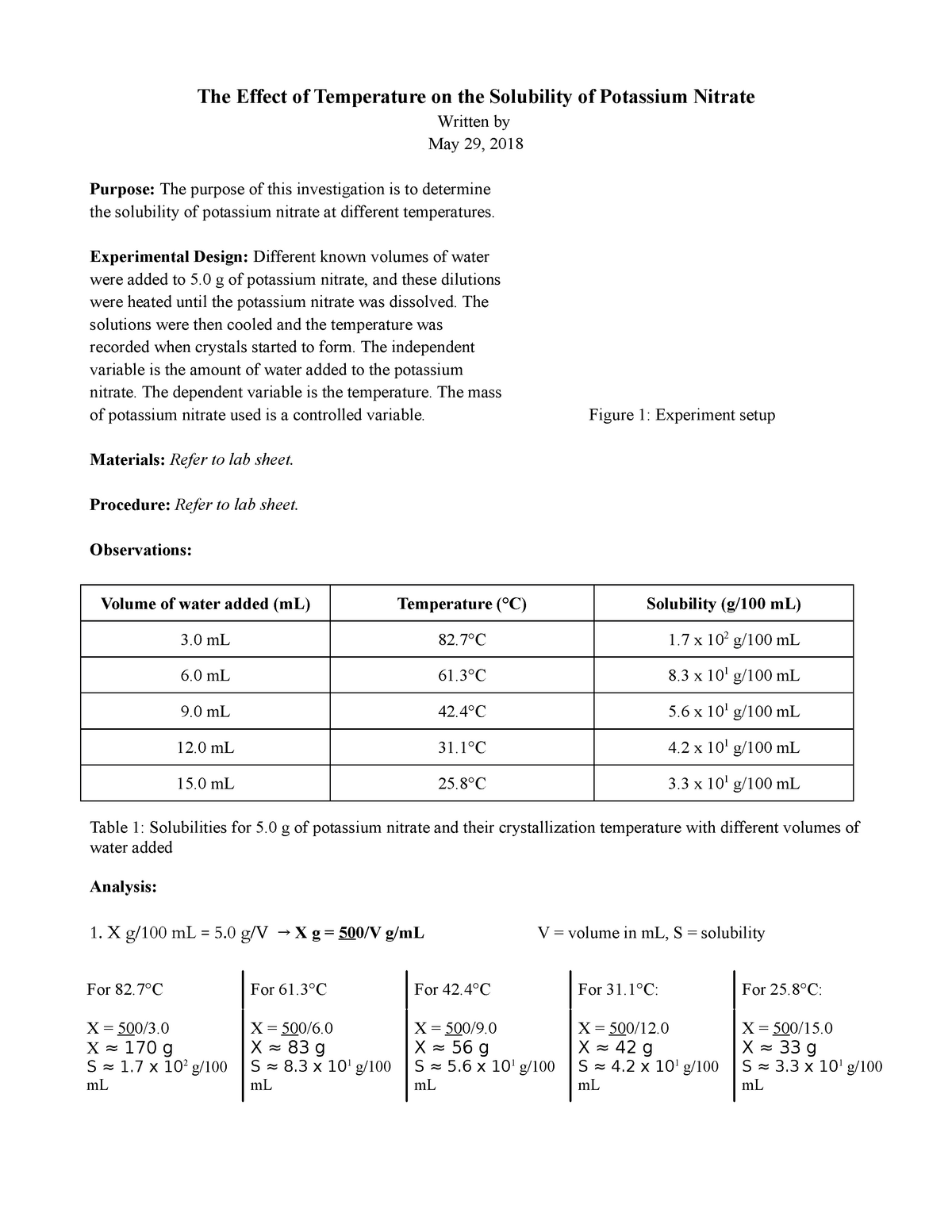
Potassium Nitrate Solubility Curve Lab The Effect of Temperature on
Lab On Solubility add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. Rank the solubility of different salts. add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. hot or cold, which water is better for soluble substances? To understand how temperature, pressure, and the presence of other solutes affect the solubility of. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. collect experimental data and create a solubility curve. Compare the number of ions in solution. Includes kit list and safety instructions. By the end of this lab, students should be able to: The solubility of a substance could depend upon the size of the solute pieces, the choice or temperature. Determine the ratio of anions and cations that create a neutral. Explore your finding from this practical into the effect of temperature on solubility.
From www.scribd.com
Lab Report (Solubility) PDF Solubility Solution Lab On Solubility collect experimental data and create a solubility curve. hot or cold, which water is better for soluble substances? Compare the number of ions in solution. Includes kit list and safety instructions. Rank the solubility of different salts. Explore your finding from this practical into the effect of temperature on solubility. students explore the chemistry behind the causes. Lab On Solubility.
From studylib.net
Sugar Solubility Lab Lab On Solubility By the end of this lab, students should be able to: students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. Explore your finding from this practical into the effect of temperature on solubility. collect experimental data and create a solubility curve. The solubility of a substance could depend upon the. Lab On Solubility.
From ctdbfredeen.blogspot.com
Lab 20 Solubility Inquiry Lab Lab On Solubility To understand how temperature, pressure, and the presence of other solutes affect the solubility of. Explore your finding from this practical into the effect of temperature on solubility. the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. The solubility of a substance could depend upon the size of the solute. Lab On Solubility.
From studylib.net
Unit 1C3 Solubility Curve Lab Lab On Solubility The solubility of a substance could depend upon the size of the solute pieces, the choice or temperature. Compare the number of ions in solution. By the end of this lab, students should be able to: students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. Rank the solubility of different salts.. Lab On Solubility.
From studylib.net
Temperature and Solubility Virtual Lab Lab On Solubility Includes kit list and safety instructions. Compare the number of ions in solution. The solubility of a substance could depend upon the size of the solute pieces, the choice or temperature. collect experimental data and create a solubility curve. Determine the ratio of anions and cations that create a neutral. the objective of this experiment is to investigate. Lab On Solubility.
From studylib.net
Solubility of a Salt Lab Lab On Solubility Explore your finding from this practical into the effect of temperature on solubility. collect experimental data and create a solubility curve. By the end of this lab, students should be able to: Compare the number of ions in solution. the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. . Lab On Solubility.
From iu.pressbooks.pub
Effect of Temperature and Solvent on Solubility IU East Experimental Lab On Solubility To understand how temperature, pressure, and the presence of other solutes affect the solubility of. By the end of this lab, students should be able to: Includes kit list and safety instructions. Compare the number of ions in solution. Determine the ratio of anions and cations that create a neutral. The solubility of a substance could depend upon the size. Lab On Solubility.
From www.youtube.com
Lab 6Physical and Chemical Solubility YouTube Lab On Solubility Rank the solubility of different salts. Determine the ratio of anions and cations that create a neutral. By the end of this lab, students should be able to: the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. Compare the number of ions in solution. Includes kit list and safety instructions.. Lab On Solubility.
From www.scribd.com
lab solubility and temperature Solubility Solution Lab On Solubility students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. Includes kit list and safety instructions. Compare the number of ions in solution. collect experimental data and create a solubility curve. Determine. Lab On Solubility.
From www.docsity.com
Solubility General Chemistry Lecture Slides Docsity Lab On Solubility students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. Compare the number of ions in solution. Rank the solubility of different salts. The solubility of a substance could depend upon the size of the solute pieces, the choice or temperature. Includes kit list and safety instructions. collect experimental data and. Lab On Solubility.
From www.youtube.com
SOLUBILITY TEST YouTube Lab On Solubility The solubility of a substance could depend upon the size of the solute pieces, the choice or temperature. Explore your finding from this practical into the effect of temperature on solubility. By the end of this lab, students should be able to: To understand how temperature, pressure, and the presence of other solutes affect the solubility of. add different. Lab On Solubility.
From studylib.net
Solubility Lab Lab On Solubility add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. By the end of this lab, students should be able to: To understand how temperature, pressure, and the presence of other solutes affect the solubility of. Includes kit list and safety instructions. Explore your finding from this practical into the effect of. Lab On Solubility.
From studylib.net
Solubility Lab Lab On Solubility collect experimental data and create a solubility curve. Includes kit list and safety instructions. Determine the ratio of anions and cations that create a neutral. Compare the number of ions in solution. To understand how temperature, pressure, and the presence of other solutes affect the solubility of. Explore your finding from this practical into the effect of temperature on. Lab On Solubility.
From nationalsportsclinics.com
Solubility lab report National Sports Clinics Lab On Solubility collect experimental data and create a solubility curve. Rank the solubility of different salts. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. hot or cold, which water is better for soluble substances? By the end of this lab, students should be able to: Compare the number of ions. Lab On Solubility.
From mycentennial.sd43.bc.ca
Determination of a Solubility Product Constant Lab 19C Matthew’s Blog Lab On Solubility To understand how temperature, pressure, and the presence of other solutes affect the solubility of. Rank the solubility of different salts. the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. By the end of this lab, students should be able to: collect experimental data and create a solubility curve.. Lab On Solubility.
From studylib.net
Solubility Curve Lab Lab On Solubility add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. Determine the ratio of anions and cations that create a neutral. Includes kit list and safety instructions. By the end of this lab, students should be able to: The solubility of a substance could depend upon the size of the solute pieces,. Lab On Solubility.
From studylib.net
Solubility Lab Lab On Solubility Determine the ratio of anions and cations that create a neutral. The solubility of a substance could depend upon the size of the solute pieces, the choice or temperature. hot or cold, which water is better for soluble substances? the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. Compare. Lab On Solubility.
From studylib.net
Solubility Rules Lab Lab On Solubility students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. hot or cold, which water is better for soluble substances? By the end of this lab, students should be able to: add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. Determine the. Lab On Solubility.
From www.youtube.com
Solubility Lab Visuals YouTube Lab On Solubility Determine the ratio of anions and cations that create a neutral. Rank the solubility of different salts. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. hot or cold, which water is better for soluble substances? add different salts to water, then watch them dissolve and achieve a dynamic. Lab On Solubility.
From julietsobel.blogspot.com
Juliet's Chemistry Blog Constructing A Solubility Curve Lab Lab On Solubility Compare the number of ions in solution. By the end of this lab, students should be able to: The solubility of a substance could depend upon the size of the solute pieces, the choice or temperature. hot or cold, which water is better for soluble substances? students explore the chemistry behind the causes and effects of acid mine. Lab On Solubility.
From www.studocu.com
lab report for solubility CHM101L Solubility Materials Obtain the Lab On Solubility add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. The solubility of a substance could depend upon the size of the solute pieces, the choice or temperature. Explore your finding from this practical into the effect of temperature on solubility. To understand how temperature, pressure, and the presence of other solutes. Lab On Solubility.
From www.scribd.com
Solubility Lab Report Template PDF Lab On Solubility Explore your finding from this practical into the effect of temperature on solubility. hot or cold, which water is better for soluble substances? the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled. Lab On Solubility.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID6260427 Lab On Solubility collect experimental data and create a solubility curve. Compare the number of ions in solution. Determine the ratio of anions and cations that create a neutral. the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. By the end of this lab, students should be able to: Rank the solubility. Lab On Solubility.
From studylib.net
Solubility Rules Lab Lab On Solubility collect experimental data and create a solubility curve. Explore your finding from this practical into the effect of temperature on solubility. Determine the ratio of anions and cations that create a neutral. add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. students explore the chemistry behind the causes and. Lab On Solubility.
From studylib.net
Solubility Curve Lab Lab On Solubility Explore your finding from this practical into the effect of temperature on solubility. Determine the ratio of anions and cations that create a neutral. By the end of this lab, students should be able to: Includes kit list and safety instructions. add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. To. Lab On Solubility.
From www.youtube.com
3 24 Solubility Virtual Lab teacher lesson YouTube Lab On Solubility To understand how temperature, pressure, and the presence of other solutes affect the solubility of. collect experimental data and create a solubility curve. the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. Rank the solubility of different salts. hot or cold, which water is better for soluble substances?. Lab On Solubility.
From www.studocu.com
Lab Report 1 Solubility Tam Huynh Partner Lindsey Campbell Prof Lab On Solubility collect experimental data and create a solubility curve. Includes kit list and safety instructions. hot or cold, which water is better for soluble substances? students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. Compare the number of ions in solution. To understand how temperature, pressure, and the presence of. Lab On Solubility.
From studylib.net
Solubility Rules Lab Varga Lab On Solubility the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. Determine the ratio of anions and cations that create a neutral. Explore your finding from this practical into the effect of temperature on solubility. To understand how temperature, pressure, and the presence of other solutes affect the solubility of. hot. Lab On Solubility.
From www.studocu.com
Potassium Nitrate Solubility Curve Lab The Effect of Temperature on Lab On Solubility add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. collect experimental data and create a solubility curve. Explore your finding from this practical into the effect of temperature on solubility. the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. To. Lab On Solubility.
From studylib.net
Instructions Lab Solubility of KNO3 Lab On Solubility add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. Compare the number of ions in solution. Determine the ratio of anions and cations that create a neutral. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. the objective of this experiment. Lab On Solubility.
From www.studocu.com
Lab Report Effects Of Temperature On Solubility Of A Salt LAB Report Lab On Solubility add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. By the end of this lab, students should be able to: The solubility of a substance could depend upon the size of the. Lab On Solubility.
From www.youtube.com
virtual solubility curve lab YouTube Lab On Solubility the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. Explore your finding from this practical into the effect of temperature on solubility. Determine the ratio of anions and cations that create a neutral. The solubility of a substance could depend upon the size of the solute pieces, the choice or. Lab On Solubility.
From studylib.net
Solubility Curve Worksheet and Lab Lab On Solubility By the end of this lab, students should be able to: add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. To understand how temperature, pressure, and the presence of other solutes affect the solubility of. Explore your finding from this practical into the effect of temperature on solubility. Determine the ratio. Lab On Solubility.
From studylib.net
LAB Precipitates and Solubility Rules Lab On Solubility Explore your finding from this practical into the effect of temperature on solubility. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. Compare the number of ions in solution. collect experimental data and create a solubility curve. add different salts to water, then watch them dissolve and achieve a. Lab On Solubility.
From surfguppy.com
Solubility Surfguppy Chemistry made easy visual learning Lab On Solubility students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. By the end of this lab, students should be able to: Rank the solubility of different salts. add different salts to water, then watch them dissolve and achieve a dynamic equilibrium with solid precipitate. collect experimental data and create a. Lab On Solubility.