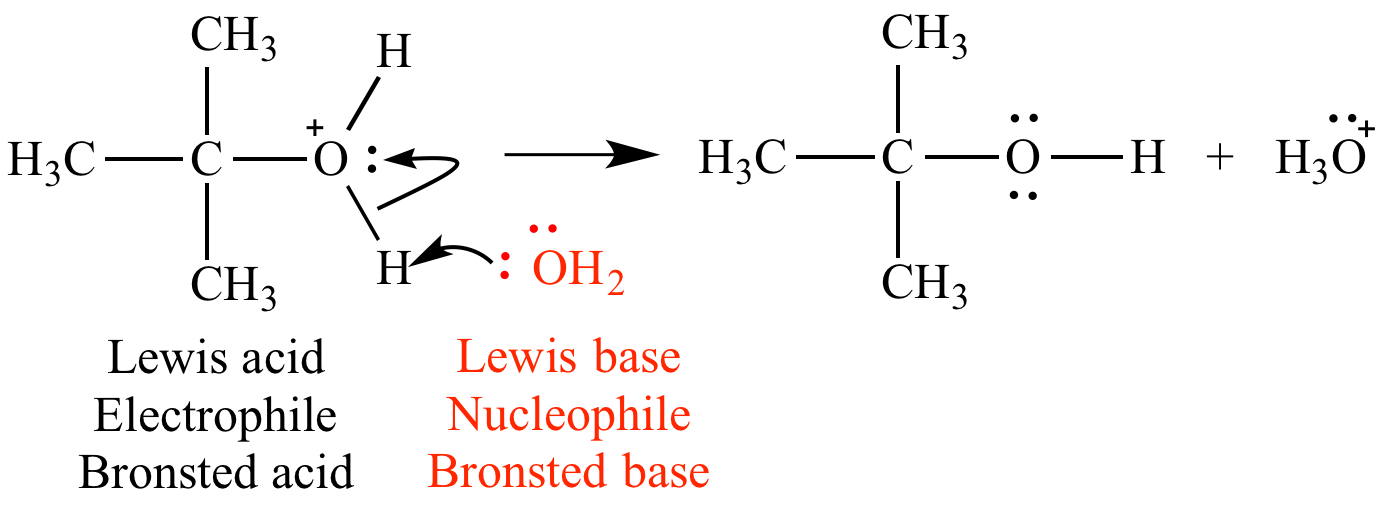What Is Meant By Lewis Base . a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. Lewis bases donate an electron pair. identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure. Lewis bases are nucleophilic meaning that they “attack” a positive. Identify a given compound as being a lewis acid or lewis base, given its lewis. A lewis acid is an electrophilic species that accepts a pair of electrons. shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. What are lewis acid and lewis base? state the lewis definition of an acid and a base.
from www.chem.ucla.edu
Identify a given compound as being a lewis acid or lewis base, given its lewis. shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. Lewis bases donate an electron pair. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. What are lewis acid and lewis base? A lewis acid is an electrophilic species that accepts a pair of electrons. Lewis bases are nucleophilic meaning that they “attack” a positive. identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure. state the lewis definition of an acid and a base.
Illustrated Glossary of Organic Chemistry Lewis acid
What Is Meant By Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. state the lewis definition of an acid and a base. shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. Lewis bases donate an electron pair. A lewis acid is an electrophilic species that accepts a pair of electrons. Identify a given compound as being a lewis acid or lewis base, given its lewis. Lewis bases are nucleophilic meaning that they “attack” a positive. identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. What are lewis acid and lewis base?
From www.youtube.com
Understanding Lewis Bases YouTube What Is Meant By Lewis Base A lewis acid is an electrophilic species that accepts a pair of electrons. state the lewis definition of an acid and a base. What are lewis acid and lewis base? shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. identify a given compound as being. What Is Meant By Lewis Base.
From denmarkgroup.illinois.edu
Lewis Base Activation of Lewis Acids Denmark Group What Is Meant By Lewis Base Lewis bases donate an electron pair. identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure. Identify a given compound as being a lewis acid or lewis base, given its lewis. state the lewis definition of an acid and a base. What are lewis acid and lewis base?. What Is Meant By Lewis Base.
From www.slideserve.com
PPT Lewis Acids & Bases PowerPoint Presentation, free download ID What Is Meant By Lewis Base state the lewis definition of an acid and a base. What are lewis acid and lewis base? Identify a given compound as being a lewis acid or lewis base, given its lewis. A lewis acid is an electrophilic species that accepts a pair of electrons. a lewis base is defined as any species that can donate a pair. What Is Meant By Lewis Base.
From www.vrogue.co
Lewis Acids And Bases vrogue.co What Is Meant By Lewis Base state the lewis definition of an acid and a base. What are lewis acid and lewis base? identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure. Identify a given compound as being a lewis acid or lewis base, given its lewis. Lewis bases donate an electron pair.. What Is Meant By Lewis Base.
From www.youtube.com
Classify the Substances as Lewis Acids or Lewis Bases Identifying What Is Meant By Lewis Base state the lewis definition of an acid and a base. What are lewis acid and lewis base? Lewis bases donate an electron pair. Identify a given compound as being a lewis acid or lewis base, given its lewis. identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure.. What Is Meant By Lewis Base.
From keithkruwmitchell.blogspot.com
Lewis Acid and Base KeithkruwMitchell What Is Meant By Lewis Base What are lewis acid and lewis base? identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. A lewis. What Is Meant By Lewis Base.
From www.ck12.org
Lewis Acids and Bases Overview ( Video ) Chemistry CK12 Foundation What Is Meant By Lewis Base Lewis bases are nucleophilic meaning that they “attack” a positive. Lewis bases donate an electron pair. A lewis acid is an electrophilic species that accepts a pair of electrons. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. What. What Is Meant By Lewis Base.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free What Is Meant By Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. A lewis acid is an electrophilic species that accepts a pair of electrons. shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert. What Is Meant By Lewis Base.
From www.meta-synthesis.com
Lewis Acid Base Interaction Matrix Chemogenesis What Is Meant By Lewis Base A lewis acid is an electrophilic species that accepts a pair of electrons. Identify a given compound as being a lewis acid or lewis base, given its lewis. Lewis bases donate an electron pair. state the lewis definition of an acid and a base. a lewis base is defined as any species that can donate a pair of. What Is Meant By Lewis Base.
From www.numerade.com
SOLVED Identify the Lewis base in this balanced equation. 6H2O + Cr3 What Is Meant By Lewis Base shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. Identify a given compound as being a lewis acid or lewis base, given its lewis. Lewis bases are nucleophilic meaning that they “attack” a positive. What are lewis acid and lewis base? identify a given compound as. What Is Meant By Lewis Base.
From www.slideserve.com
PPT Lewis Acids & Bases PowerPoint Presentation, free download ID What Is Meant By Lewis Base shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. Lewis bases are nucleophilic meaning that they “attack” a positive. A lewis acid is an electrophilic species that accepts a pair of electrons. What are lewis acid and lewis base? identify a given compound as being a. What Is Meant By Lewis Base.
From d3f6gjnauy613m.cloudfront.net
Basen • Definition und Erklärung · [mit Video] What Is Meant By Lewis Base identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure. A lewis acid is an electrophilic species that accepts a pair of electrons. What are lewis acid and lewis base? Lewis bases are nucleophilic meaning that they “attack” a positive. state the lewis definition of an acid and. What Is Meant By Lewis Base.
From denmarkgroup.illinois.edu
Lewis Base Activation of Lewis Acids Denmark Group What Is Meant By Lewis Base What are lewis acid and lewis base? shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. Lewis bases are nucleophilic meaning that they “attack” a positive. state the lewis definition of an acid and a base. A lewis acid is an electrophilic species that accepts a. What Is Meant By Lewis Base.
From www.slideserve.com
PPT Calculating Percent Ionization PowerPoint Presentation ID604587 What Is Meant By Lewis Base identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure. What are lewis acid and lewis base? Lewis bases donate an electron pair. A lewis acid is an electrophilic species that accepts a pair of electrons. a lewis base is defined as any species that can donate a. What Is Meant By Lewis Base.
From www.numerade.com
SOLVEDExplain or illustrate by an example what is meant by identifying What Is Meant By Lewis Base identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure. Identify a given compound as being a lewis acid or lewis base, given its lewis. Lewis bases donate an electron pair. shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton. What Is Meant By Lewis Base.
From www.sliderbase.com
Acids and Bases The Lewis Definition What Is Meant By Lewis Base A lewis acid is an electrophilic species that accepts a pair of electrons. Lewis bases donate an electron pair. identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid. What Is Meant By Lewis Base.
From www.u-helmich.de
LewisBase What Is Meant By Lewis Base state the lewis definition of an acid and a base. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. A lewis acid is an electrophilic species that accepts a pair of electrons. shortly after bronsted and lowry. What Is Meant By Lewis Base.
From www.slideserve.com
PPT Lewis Acids & Bases PowerPoint Presentation, free download ID What Is Meant By Lewis Base shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. state the lewis definition of an acid. What Is Meant By Lewis Base.
From www.pinterest.com
Lewis Acids and Bases Lewis acids and bases, Organic reactions, Chemistry What Is Meant By Lewis Base state the lewis definition of an acid and a base. Identify a given compound as being a lewis acid or lewis base, given its lewis. Lewis bases donate an electron pair. What are lewis acid and lewis base? identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure.. What Is Meant By Lewis Base.
From ecampusontario.pressbooks.pub
5.6 Lewis Acids & Bases General Chemistry for GeeGees What Is Meant By Lewis Base What are lewis acid and lewis base? state the lewis definition of an acid and a base. A lewis acid is an electrophilic species that accepts a pair of electrons. shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. Lewis bases are nucleophilic meaning that they. What Is Meant By Lewis Base.
From www.slideserve.com
PPT The Lewis AcidBase Model PowerPoint Presentation, free download What Is Meant By Lewis Base What are lewis acid and lewis base? identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure. Identify a given compound as being a lewis acid or lewis base, given its lewis. Lewis bases are nucleophilic meaning that they “attack” a positive. A lewis acid is an electrophilic species. What Is Meant By Lewis Base.
From byjus.com
the strongest lewis base is i.nh3 ii.nf3 iii.nh2oh iv.n(sih3) What Is Meant By Lewis Base A lewis acid is an electrophilic species that accepts a pair of electrons. What are lewis acid and lewis base? a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. Lewis bases donate an electron pair. identify a given. What Is Meant By Lewis Base.
From facts.net
17 Astounding Facts About Lewis AcidBase Theory What Is Meant By Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. state the lewis definition of an acid and a base. Lewis bases are nucleophilic meaning that they “attack” a positive. What are lewis acid and lewis base? A lewis. What Is Meant By Lewis Base.
From www.ck12.org
Lewis Acids and Bases Example 1 ( Video ) Chemistry CK12 Foundation What Is Meant By Lewis Base What are lewis acid and lewis base? identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. A lewis. What Is Meant By Lewis Base.
From www.meta-synthesis.com
Lewis Acid Base Interaction Matrix Chemogenesis What Is Meant By Lewis Base Lewis bases are nucleophilic meaning that they “attack” a positive. Identify a given compound as being a lewis acid or lewis base, given its lewis. state the lewis definition of an acid and a base. Lewis bases donate an electron pair. A lewis acid is an electrophilic species that accepts a pair of electrons. a lewis base is. What Is Meant By Lewis Base.
From www.animalia-life.club
Lewis Acid Base Reaction What Is Meant By Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. Lewis bases donate an electron pair. state the lewis definition of an acid and a base. Lewis bases are nucleophilic meaning that they “attack” a positive. What are lewis. What Is Meant By Lewis Base.
From chemistryguru.com.sg
Three Definitions of Acids and Bases What Is Meant By Lewis Base Lewis bases donate an electron pair. Lewis bases are nucleophilic meaning that they “attack” a positive. What are lewis acid and lewis base? state the lewis definition of an acid and a base. A lewis acid is an electrophilic species that accepts a pair of electrons. Identify a given compound as being a lewis acid or lewis base, given. What Is Meant By Lewis Base.
From www.slideserve.com
PPT Lewis Acids, Lewis Bases, and Curvy Arrows PowerPoint What Is Meant By Lewis Base shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. Lewis bases are nucleophilic meaning that they “attack” a positive. A lewis acid is an electrophilic species that accepts a pair of electrons. Lewis bases donate an electron pair. state the lewis definition of an acid and. What Is Meant By Lewis Base.
From www.slideserve.com
PPT Lewis Acids & Bases PowerPoint Presentation, free download ID What Is Meant By Lewis Base A lewis acid is an electrophilic species that accepts a pair of electrons. Lewis bases donate an electron pair. shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. identify a given compound as being a lewis acid or lewis base, given its lewis structure or its. What Is Meant By Lewis Base.
From www.chemistrysteps.com
Lewis Acids and Bases Chemistry Steps What Is Meant By Lewis Base shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. Lewis bases are nucleophilic meaning that they “attack” a positive. identify a given compound as being a lewis acid or lewis base, given its lewis structure or its kekulé structure. Identify a given compound as being a. What Is Meant By Lewis Base.
From www.youtube.com
Lewis Acids & Lewis Bases Introduction YouTube What Is Meant By Lewis Base Lewis bases are nucleophilic meaning that they “attack” a positive. What are lewis acid and lewis base? a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. identify a given compound as being a lewis acid or lewis base,. What Is Meant By Lewis Base.
From www.meta-synthesis.com
Lewis Acid Base Reaction Chemistry Chemogenesis What Is Meant By Lewis Base shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. state the lewis definition of an acid and a base. Lewis bases are nucleophilic meaning that they “attack” a positive. identify a given compound as being a lewis acid or lewis base, given its lewis structure. What Is Meant By Lewis Base.
From sciencenotes.org
Lewis Acid and Base Theory What Is Meant By Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. state the lewis definition of an acid and a base. identify a given compound as being a lewis acid or lewis base, given its lewis structure or its. What Is Meant By Lewis Base.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Lewis acid What Is Meant By Lewis Base shortly after bronsted and lowry proposed their definition of acids and bases, the american chemist gilbert newton lewis, building upon his. Lewis bases are nucleophilic meaning that they “attack” a positive. state the lewis definition of an acid and a base. What are lewis acid and lewis base? Lewis bases donate an electron pair. Identify a given compound. What Is Meant By Lewis Base.
From www.slideserve.com
PPT Chapter 14 PowerPoint Presentation, free download ID4280814 What Is Meant By Lewis Base Identify a given compound as being a lewis acid or lewis base, given its lewis. What are lewis acid and lewis base? state the lewis definition of an acid and a base. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a. What Is Meant By Lewis Base.