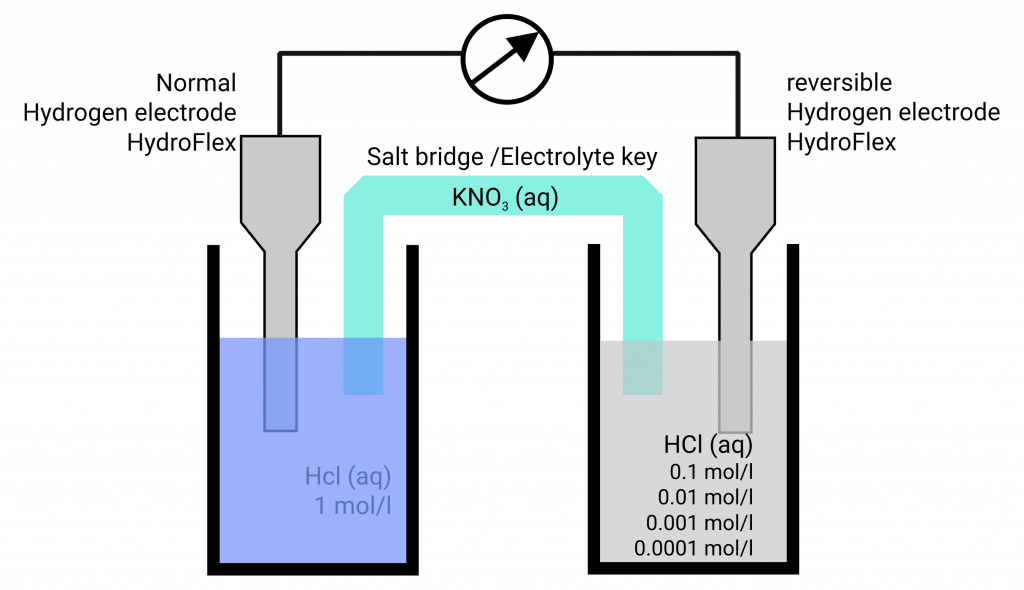
Standard Hydrogen Electrode Normal . In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which forms the. The structure of the standard hydrogen electrode is. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies the pressure. Electrode (she) and reversible hydrogen electrode (rhe)? What are normal hydrogen electrode (nhe), standard hydrogen.
from gaskatel.de
The structure of the standard hydrogen electrode is. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies the pressure. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which forms the. What are normal hydrogen electrode (nhe), standard hydrogen. In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. Electrode (she) and reversible hydrogen electrode (rhe)?
Usage of the hydrogen electrodes HydroFlex and MiniHydroFlex Gaskatel
Standard Hydrogen Electrode Normal Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies the pressure. What are normal hydrogen electrode (nhe), standard hydrogen. In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which forms the. The structure of the standard hydrogen electrode is. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. Electrode (she) and reversible hydrogen electrode (rhe)? Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies the pressure.
From www.youtube.com
Standard hydrogen electrode SHE Normal hydrogen electrode (NHE Standard Hydrogen Electrode Normal What are normal hydrogen electrode (nhe), standard hydrogen. In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which forms the. This is a reference electrode to which all electrodes are calculated in terms. Standard Hydrogen Electrode Normal.
From www.youtube.com
Standard Hydrogen Electrode (SHE) Normal Hydrogen Electrode (NHE Standard Hydrogen Electrode Normal What are normal hydrogen electrode (nhe), standard hydrogen. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies. Standard Hydrogen Electrode Normal.
From www.pinterest.com
Standard Hydrogen Electrode in 2020 Electron configuration, Ideal Standard Hydrogen Electrode Normal Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies the pressure. This is a reference electrode to. Standard Hydrogen Electrode Normal.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using Standard Hydrogen Electrode Normal The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which forms the. In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. What are normal hydrogen electrode. Standard Hydrogen Electrode Normal.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Standard Hydrogen Electrode Normal This is a reference electrode to which all electrodes are calculated in terms of electrode potential. In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. The structure of the standard hydrogen electrode is. What are normal hydrogen electrode (nhe), standard hydrogen. The standard hydrogen electrode (abbreviated she), also called. Standard Hydrogen Electrode Normal.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use Standard Hydrogen Electrode Normal What are normal hydrogen electrode (nhe), standard hydrogen. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies. Standard Hydrogen Electrode Normal.
From www.studypool.com
SOLUTION Standard hydrogen electrode she or normal Studypool Standard Hydrogen Electrode Normal Electrode (she) and reversible hydrogen electrode (rhe)? The structure of the standard hydrogen electrode is. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any. Standard Hydrogen Electrode Normal.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Normal Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies the pressure. The standard hydrogen electrode (abbreviated she),. Standard Hydrogen Electrode Normal.
From www.shutterstock.com
Standard Hydrogen Electrode Diagram Scientific Vector เวกเตอร์สต็อก Standard Hydrogen Electrode Normal Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies the pressure. In electrochemistry, the standard hydrogen electrode,. Standard Hydrogen Electrode Normal.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID5410570 Standard Hydrogen Electrode Normal Electrode (she) and reversible hydrogen electrode (rhe)? This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The structure of the standard hydrogen electrode is. In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. Although the standard hydrogen electrode is one of the. Standard Hydrogen Electrode Normal.
From www.vrogue.co
Hydrogen Standard Hydrogen Electrode vrogue.co Standard Hydrogen Electrode Normal Electrode (she) and reversible hydrogen electrode (rhe)? Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies the. Standard Hydrogen Electrode Normal.
From gaskatel.de
Reference electrode Gaskatel Standard Hydrogen Electrode Normal In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. Electrode (she) and reversible hydrogen electrode (rhe)? The structure of the standard hydrogen electrode is. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. Although the standard hydrogen electrode is one of the. Standard Hydrogen Electrode Normal.
From www.researchgate.net
Absolute and relative (respect to Standard Hydrogen Electrode, SHE Standard Hydrogen Electrode Normal Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies the pressure. What are normal hydrogen electrode (nhe),. Standard Hydrogen Electrode Normal.
From brainly.in
what is standard hydrogen electrode draw Brainly.in Standard Hydrogen Electrode Normal This is a reference electrode to which all electrodes are calculated in terms of electrode potential. Electrode (she) and reversible hydrogen electrode (rhe)? Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the. Standard Hydrogen Electrode Normal.
From stock.adobe.com
Vetor de A Standard Hydrogen Electrode or SHE is an electrode that Standard Hydrogen Electrode Normal The structure of the standard hydrogen electrode is. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies. Standard Hydrogen Electrode Normal.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Normal Electrode (she) and reversible hydrogen electrode (rhe)? The structure of the standard hydrogen electrode is. What are normal hydrogen electrode (nhe), standard hydrogen. The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which forms the. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential. Standard Hydrogen Electrode Normal.
From users.highland.edu
Standard Potentials Standard Hydrogen Electrode Normal In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the. Standard Hydrogen Electrode Normal.
From www.studypool.com
SOLUTION Standard hydrogen electrode she or normal Studypool Standard Hydrogen Electrode Normal In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. The structure of the standard hydrogen electrode is. Electrode (she) and reversible hydrogen electrode (rhe)? What are normal hydrogen electrode (nhe), standard hydrogen. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The. Standard Hydrogen Electrode Normal.
From www.researchgate.net
(PDF) What are Normal Hydrogen Electrode (NHE), Standard Hydrogen Standard Hydrogen Electrode Normal Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies the pressure. The standard hydrogen electrode (abbreviated she),. Standard Hydrogen Electrode Normal.
From gaskatel.de
Usage of the hydrogen electrodes HydroFlex and MiniHydroFlex Gaskatel Standard Hydrogen Electrode Normal This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The structure of the standard hydrogen electrode is. The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which forms the. Electrode (she) and reversible hydrogen electrode (rhe)? In electrochemistry, the standard hydrogen electrode, is a redox electrode. Standard Hydrogen Electrode Normal.
From www.studypool.com
SOLUTION Standard hydrogen electrode she or normal Studypool Standard Hydrogen Electrode Normal What are normal hydrogen electrode (nhe), standard hydrogen. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies. Standard Hydrogen Electrode Normal.
From byjus.com
What is standard hydrogen electrode? Standard Hydrogen Electrode Normal In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. Electrode (she) and reversible hydrogen electrode (rhe)? Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its. Standard Hydrogen Electrode Normal.
From cider.uoregon.edu
Standard Hydrogen Electrode (SHE) Simulation and Animation AACT CIDER Standard Hydrogen Electrode Normal What are normal hydrogen electrode (nhe), standard hydrogen. The structure of the standard hydrogen electrode is. The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which forms the. Electrode (she) and reversible hydrogen electrode (rhe)? This is a reference electrode to which all electrodes are calculated in terms of electrode potential. Although the. Standard Hydrogen Electrode Normal.
From www.slideserve.com
PPT Oxidation/Reduction Reactions In Cells PowerPoint Presentation Standard Hydrogen Electrode Normal Electrode (she) and reversible hydrogen electrode (rhe)? This is a reference electrode to which all electrodes are calculated in terms of electrode potential. What are normal hydrogen electrode (nhe), standard hydrogen. In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. The standard hydrogen electrode (abbreviated she), also called normal. Standard Hydrogen Electrode Normal.
From facts.net
16 Unbelievable Facts About Standard Electrode Potential Standard Hydrogen Electrode Normal The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which forms the. In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. What are normal hydrogen electrode. Standard Hydrogen Electrode Normal.
From www.slideserve.com
PPT Electrochemical Theory PowerPoint Presentation, free download Standard Hydrogen Electrode Normal Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen gas are unit, any temperature variation (and especially its increase) modifies the pressure. The structure of the standard hydrogen. Standard Hydrogen Electrode Normal.
From dohsdocfeco.blob.core.windows.net
Facts About Standard Hydrogen Electrode at Troy Williams blog Standard Hydrogen Electrode Normal This is a reference electrode to which all electrodes are calculated in terms of electrode potential. Electrode (she) and reversible hydrogen electrode (rhe)? Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the. Standard Hydrogen Electrode Normal.
From www.studypool.com
SOLUTION Standard hydrogen electrode she or normal Studypool Standard Hydrogen Electrode Normal What are normal hydrogen electrode (nhe), standard hydrogen. The structure of the standard hydrogen electrode is. In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. Electrode (she) and reversible hydrogen electrode (rhe)? The. Standard Hydrogen Electrode Normal.
From www.vrogue.co
Hydrogen Standard Hydrogen Electrode vrogue.co Standard Hydrogen Electrode Normal In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. Electrode (she) and reversible hydrogen electrode (rhe)? What are normal hydrogen electrode (nhe), standard hydrogen. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The standard hydrogen electrode (abbreviated she), also called normal. Standard Hydrogen Electrode Normal.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Standard Hydrogen Electrode Normal In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. Electrode (she) and reversible hydrogen electrode (rhe)? The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which forms the. Although the standard hydrogen electrode is one of the most fundamental concepts of physical. Standard Hydrogen Electrode Normal.
From scienceinfo.com
What is Standard Hydrogen Electrode? Standard Hydrogen Electrode Normal Electrode (she) and reversible hydrogen electrode (rhe)? This is a reference electrode to which all electrodes are calculated in terms of electrode potential. In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which. Standard Hydrogen Electrode Normal.
From www.doubtnut.com
Describe the construction and working of the standard hydrogen electr Standard Hydrogen Electrode Normal The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which forms the. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of the hydrogen. Standard Hydrogen Electrode Normal.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Normal The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which forms the. The structure of the standard hydrogen electrode is. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. Electrode (she) and reversible hydrogen electrode (rhe)? In electrochemistry, the standard hydrogen electrode, is a redox electrode. Standard Hydrogen Electrode Normal.
From mavink.com
Hydrogen Electrode Diagram Standard Hydrogen Electrode Normal The structure of the standard hydrogen electrode is. Electrode (she) and reversible hydrogen electrode (rhe)? This is a reference electrode to which all electrodes are calculated in terms of electrode potential. What are normal hydrogen electrode (nhe), standard hydrogen. In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation. Although. Standard Hydrogen Electrode Normal.
From dxoqyggvw.blob.core.windows.net
Electrode Nomenclature Meaning at Paul Jones blog Standard Hydrogen Electrode Normal The structure of the standard hydrogen electrode is. What are normal hydrogen electrode (nhe), standard hydrogen. The standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox electrode which forms the. Electrode (she) and reversible hydrogen electrode (rhe)? In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale. Standard Hydrogen Electrode Normal.