Aluminum Oxide In Water . in what way and in what form does aluminum react with water? aluminium oxide is amphoteric. aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. Aluminum metal rapidly develops a thin layer of aluminum oxide of. It has reactions as both a base and an acid. It is insoluble in water and has many applications in ceramics, catalysts, and water purification. this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. in this video we will describe the equation al2o3 + h2o and write what. aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases.
from aluminiumoxidekirikaza.blogspot.com
It is insoluble in water and has many applications in ceramics, catalysts, and water purification. in what way and in what form does aluminum react with water? this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. It has reactions as both a base and an acid. aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. aluminium oxide is amphoteric. aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. Aluminum metal rapidly develops a thin layer of aluminum oxide of. in this video we will describe the equation al2o3 + h2o and write what.
Aluminium Oxide Reaction Of Aluminium Oxide With Sulphuric Acid
Aluminum Oxide In Water in what way and in what form does aluminum react with water? this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. It is insoluble in water and has many applications in ceramics, catalysts, and water purification. aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. It has reactions as both a base and an acid. aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. in what way and in what form does aluminum react with water? Aluminum metal rapidly develops a thin layer of aluminum oxide of. in this video we will describe the equation al2o3 + h2o and write what. aluminium oxide is amphoteric.
From shop.jhtechnologies.com
Aluminum Oxide Powder, 3um, 5lb JH Technologies Aluminum Oxide In Water It is insoluble in water and has many applications in ceramics, catalysts, and water purification. in this video we will describe the equation al2o3 + h2o and write what. in what way and in what form does aluminum react with water? It has reactions as both a base and an acid. aluminium oxide (al2o3) is an amphoteric. Aluminum Oxide In Water.
From changecominon.blogspot.com
Melting Temp Of Aluminum change comin Aluminum Oxide In Water in this video we will describe the equation al2o3 + h2o and write what. aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. It is insoluble in water and has many applications in ceramics, catalysts, and water purification. in what way and in what form does aluminum. Aluminum Oxide In Water.
From mammothmemory.net
Aluminium reaction to hydrochloric acid is slow to start Aluminum Oxide In Water in this video we will describe the equation al2o3 + h2o and write what. It has reactions as both a base and an acid. It is insoluble in water and has many applications in ceramics, catalysts, and water purification. Aluminum metal rapidly develops a thin layer of aluminum oxide of. in what way and in what form does. Aluminum Oxide In Water.
From www.us-nano.com
Aluminum Oxide Al2O3 Nanopowder / Nanoparticles Water Dispersion (Alpha Aluminum Oxide In Water Aluminum metal rapidly develops a thin layer of aluminum oxide of. aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. It is insoluble in water and has many applications in ceramics, catalysts, and water purification. in what way and in what form does aluminum react with water? this insoluble material in water is. Aluminum Oxide In Water.
From www.youtube.com
How to write the equation for Al2O3 + H2O (Aluminum oxide + Water Aluminum Oxide In Water this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. in this video we. Aluminum Oxide In Water.
From www.pngegg.com
Aluminium oxide Aluminium powder Water, water, material, metal png PNGEgg Aluminum Oxide In Water Aluminum metal rapidly develops a thin layer of aluminum oxide of. It is insoluble in water and has many applications in ceramics, catalysts, and water purification. aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. this insoluble material in water is extremely stable and resistant to many acids,. Aluminum Oxide In Water.
From www.youtube.com
What happens when Sodium Hydroxide and Aluminium Reacts Aluminum Oxide In Water this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. Aluminum metal rapidly develops a thin layer of aluminum oxide of. in this video we will describe the equation al2o3 + h2o and write what. It has reactions as both a base and an acid. aluminium oxide. Aluminum Oxide In Water.
From www.slideserve.com
PPT Unit 8 Chemical Reactions PowerPoint Presentation, free download Aluminum Oxide In Water It has reactions as both a base and an acid. aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. in what way and in what form does aluminum react with water? in this video we will describe the equation al2o3 + h2o and write what. Aluminum metal rapidly develops a thin layer of. Aluminum Oxide In Water.
From www.slideserve.com
PPT Aluminium Oxide for Column Chromatography PowerPoint Presentation Aluminum Oxide In Water Aluminum metal rapidly develops a thin layer of aluminum oxide of. aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. aluminium oxide is amphoteric. in what way and in what form does. Aluminum Oxide In Water.
From metabunk.org
Bunk Hygroscopic Aluminum Oxide Cloud Seeding Metabunk Aluminum Oxide In Water in what way and in what form does aluminum react with water? aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. in this video we will describe the equation al2o3 + h2o and write what. It has reactions as both a base and an acid. this insoluble material in water is extremely. Aluminum Oxide In Water.
From www.youtube.com
Lewis Dot Structure for Al2O3 (Aluminum Oxide) YouTube Aluminum Oxide In Water aluminium oxide is amphoteric. in this video we will describe the equation al2o3 + h2o and write what. It is insoluble in water and has many applications in ceramics, catalysts, and water purification. It has reactions as both a base and an acid. aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. Aluminum. Aluminum Oxide In Water.
From nanografi.com
Aluminum Oxide (Al2O3) Nanopowder/Nanoparticles Dispersion in Water Aluminum Oxide In Water aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. Aluminum metal rapidly develops a thin layer of aluminum oxide of. in what way and in what form does aluminum react with water? It is insoluble in water and has many applications in ceramics, catalysts, and water purification. this insoluble material in water is. Aluminum Oxide In Water.
From www.researchgate.net
(PDF) The Effect of Threaded Rod Inserts and Aluminum OxideWater Based Aluminum Oxide In Water aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. It is insoluble in water and has many applications in ceramics, catalysts, and water purification. Aluminum metal rapidly develops a thin layer of aluminum oxide of. in what way and in what form does aluminum react with water? aluminum oxide, with the chemical formula. Aluminum Oxide In Water.
From shop.buehler.com
Aluminum Oxide Powder, 5µm, 5lb Buehler Aluminum Oxide In Water in what way and in what form does aluminum react with water? aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. It has reactions as both a base and an acid. Aluminum metal rapidly. Aluminum Oxide In Water.
From www.mdpi.com
Materials Free FullText Influence of the Composition, Structure Aluminum Oxide In Water It has reactions as both a base and an acid. in what way and in what form does aluminum react with water? aluminium oxide is amphoteric. this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. in this video we will describe the equation al2o3 +. Aluminum Oxide In Water.
From www.indiamart.com
Aluminum Oxide nanoparticles Dispersion in Water, Liquid at Rs 650/kg Aluminum Oxide In Water It has reactions as both a base and an acid. in this video we will describe the equation al2o3 + h2o and write what. in what way and in what form does aluminum react with water? this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. . Aluminum Oxide In Water.
From www.alamy.com
Vessel, aluminium oxide, watercontaining, powder, medicine Aluminum Oxide In Water aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. It is insoluble in water and has many applications in ceramics, catalysts, and water purification. aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. aluminium oxide is amphoteric. in this video we will. Aluminum Oxide In Water.
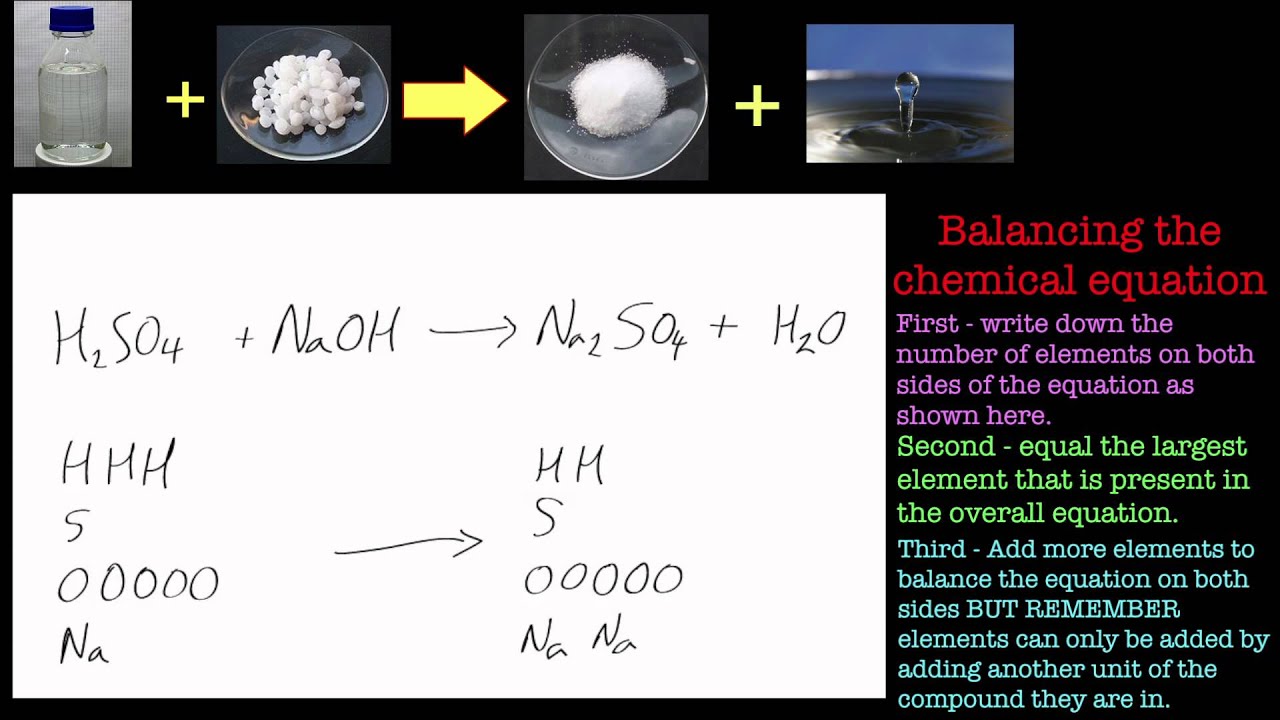
From www.slideserve.com
PPT Balancing Chemical Equations PowerPoint Presentation, free Aluminum Oxide In Water aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. Aluminum metal rapidly develops a thin layer of aluminum oxide of. aluminium oxide is amphoteric. It is insoluble in water and has many applications in. Aluminum Oxide In Water.
From in.pinterest.com
Column Chromatography Adsorbents Silica Gel and Aluminium Oxide Aluminum Oxide In Water this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. in this video we will describe the equation al2o3 + h2o and write what. in what way and in what form does aluminum react with water? aluminium oxide (al2o3) is an amphoteric substance that reacts with. Aluminum Oxide In Water.
From www.pw.live
Aluminium Oxide Formula, Structure, Properties, Uses Aluminum Oxide In Water It is insoluble in water and has many applications in ceramics, catalysts, and water purification. aluminium oxide is amphoteric. in this video we will describe the equation al2o3 + h2o and write what. in what way and in what form does aluminum react with water? It has reactions as both a base and an acid. aluminum. Aluminum Oxide In Water.
From mammothmemory.net
Aluminium reaction to hydrochloric acid is slow to start Aluminum Oxide In Water aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. in what way and in what form does aluminum react with water? It is insoluble in water and has many applications in ceramics, catalysts, and. Aluminum Oxide In Water.
From www.researchgate.net
a. The surface of aluminum oxide. Download Scientific Diagram Aluminum Oxide In Water in what way and in what form does aluminum react with water? It has reactions as both a base and an acid. in this video we will describe the equation al2o3 + h2o and write what. this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. . Aluminum Oxide In Water.
From newatlas.com
Aluminum oxide coatings move like liquids to combat corrosion Aluminum Oxide In Water It is insoluble in water and has many applications in ceramics, catalysts, and water purification. It has reactions as both a base and an acid. this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. Aluminum metal rapidly develops a thin layer of aluminum oxide of. aluminum oxide,. Aluminum Oxide In Water.
From www.nagwa.com
Question Video Describing the Oxide of Aluminum Nagwa Aluminum Oxide In Water It is insoluble in water and has many applications in ceramics, catalysts, and water purification. aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. Aluminum metal rapidly develops. Aluminum Oxide In Water.
From www.youtube.com
Al2O3+H2SO4=Al2(SO4)3+H2O Balanced EquationAluminum oxide+Sulphuric Aluminum Oxide In Water aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. Aluminum metal rapidly develops a thin layer of aluminum oxide of. in what way and in what form does aluminum react with water? It is insoluble in water and has many applications in ceramics, catalysts, and water purification. . Aluminum Oxide In Water.
From www.researchgate.net
Aluminum oxide nanoparticles. Download Scientific Diagram Aluminum Oxide In Water It is insoluble in water and has many applications in ceramics, catalysts, and water purification. in this video we will describe the equation al2o3 + h2o and write what. aluminium oxide is amphoteric. It has reactions as both a base and an acid. in what way and in what form does aluminum react with water? this. Aluminum Oxide In Water.
From www.researchgate.net
Schematic twodimensional representation of aluminum oxide structures Aluminum Oxide In Water this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. aluminium oxide is amphoteric. aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. in this video we will describe the equation al2o3 + h2o and write what. in what way and. Aluminum Oxide In Water.
From cartoondealer.com
Hydrated Aluminium Oxides Formula On Waterdrop Background RoyaltyFree Aluminum Oxide In Water aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. It is insoluble in water and has many applications in ceramics, catalysts, and water purification. in this video we will describe the equation al2o3 + h2o and write what. aluminium oxide is amphoteric. in what way and. Aluminum Oxide In Water.
From www.youtube.com
Writing the Formula for Aluminum Oxide YouTube Aluminum Oxide In Water aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. It is insoluble in water and has many applications in ceramics, catalysts, and water purification. It has reactions as both a base and an acid. in this video we will describe the equation al2o3 + h2o and write what.. Aluminum Oxide In Water.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Reaction Of Aluminium Oxide With Sulphuric Acid Aluminum Oxide In Water aluminium oxide is amphoteric. in this video we will describe the equation al2o3 + h2o and write what. It has reactions as both a base and an acid. this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. aluminum oxide, with the chemical formula al2o3 a. Aluminum Oxide In Water.
From www.youtube.com
Balance Al + H2O = Al(OH)3 + H2 (Aluminum and Water) YouTube Aluminum Oxide In Water It has reactions as both a base and an acid. in this video we will describe the equation al2o3 + h2o and write what. in what way and in what form does aluminum react with water? this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. . Aluminum Oxide In Water.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Aluminium Oxide React With Water Aluminum Oxide In Water aluminium oxide is amphoteric. aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. in what way and in what form does aluminum react with water? aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. this insoluble material in water is extremely. Aluminum Oxide In Water.
From chem.libretexts.org
Chapter 19.6 Corrosion Chemistry LibreTexts Aluminum Oxide In Water It is insoluble in water and has many applications in ceramics, catalysts, and water purification. aluminium oxide (al2o3) is an amphoteric substance that reacts with acids and bases. aluminium oxide is amphoteric. aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. in what way and in. Aluminum Oxide In Water.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Aluminium Oxide And Water Aluminum Oxide In Water this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. in this video we will describe the equation al2o3 + h2o and write what. aluminium oxide is amphoteric. in what way and in what form does aluminum react with water? Aluminum metal rapidly develops a thin. Aluminum Oxide In Water.
From aluminiumoxidekirikaza.blogspot.com
Aluminium Oxide Aluminium Oxide With Water Aluminum Oxide In Water aluminum oxide, with the chemical formula al2o3 a l 2 o 3, is an amphoteric oxide and is commonly. It is insoluble in water and has many applications in ceramics, catalysts, and water purification. this insoluble material in water is extremely stable and resistant to many acids, but it is soluble in alkaline solutions. in what way. Aluminum Oxide In Water.