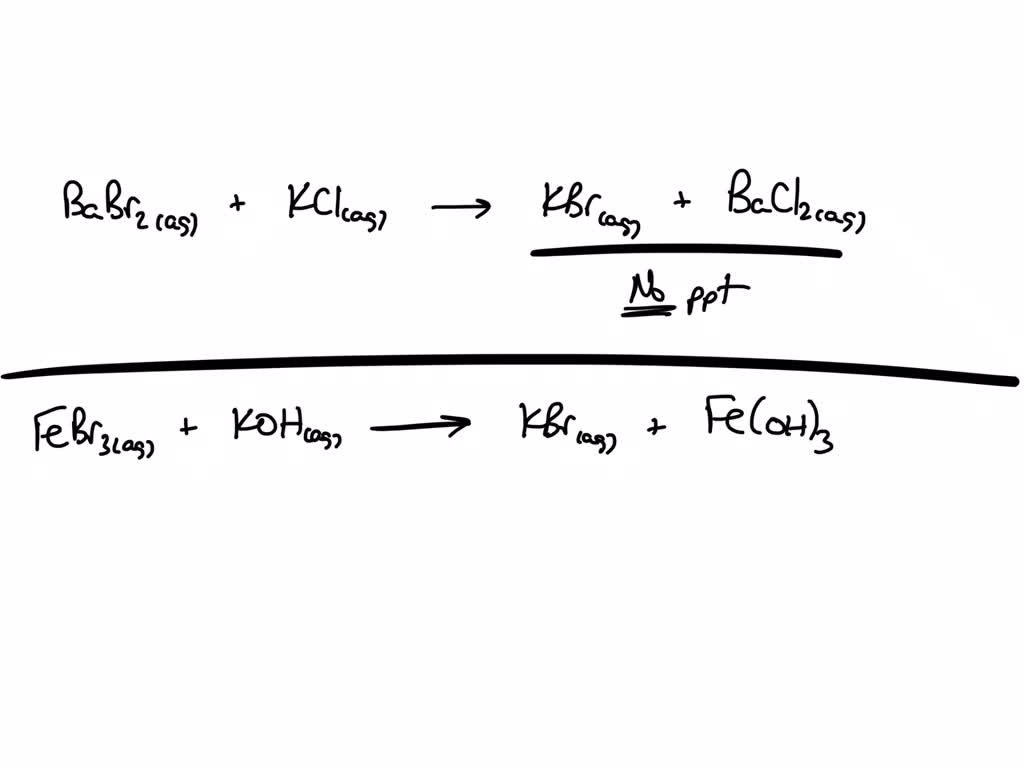
Barium Bromide Is A Precipitate . a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Babr 2 (aq) + so. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. Sodium chloride, sodium hydroxide, or. the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate.
from www.numerade.com
in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Babr 2 (aq) + so. Sodium chloride, sodium hydroxide, or. the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous.
SOLVED Does a solution A solution B precipitate form when A and B are
Barium Bromide Is A Precipitate the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. Sodium chloride, sodium hydroxide, or. Babr 2 (aq) + so. the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: which solution could be used to precipitate the barium ion, ba 2+, in a water sample: solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate.
From testbook.com
Barium bromide formula Its chemical formula, structure and uses Barium Bromide Is A Precipitate it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. watch this video to learn about precipitation reactions, a type of chemical reaction that produces. Barium Bromide Is A Precipitate.
From www.numerade.com
SOLVED Texts answer 18 Questions 1. How could you tell the Barium Bromide Is A Precipitate solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate. a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. . Barium Bromide Is A Precipitate.
From informacionpublica.svet.gob.gt
Barium Bromide Formula informacionpublica.svet.gob.gt Barium Bromide Is A Precipitate Babr 2 (aq) + so. it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of. Barium Bromide Is A Precipitate.
From www.numerade.com
SOLVEDprecipitate will form enter its empirical formula in the last Barium Bromide Is A Precipitate a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate. the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: it reacts with the sulphate. Barium Bromide Is A Precipitate.
From www.funcmater.com
buy Barium Bromide Powder price FUNCMATER Barium Bromide Is A Precipitate it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: watch this video to learn. Barium Bromide Is A Precipitate.
From fphoto.photoshelter.com
science chemistry precipitation reaction barium chromate Fundamental Barium Bromide Is A Precipitate Sodium chloride, sodium hydroxide, or. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to. Barium Bromide Is A Precipitate.
From www.youtube.com
How to write chemical formula Barium bromideChemical formula Barium Barium Bromide Is A Precipitate Babr 2 (aq) + so. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. Sodium chloride, sodium hydroxide, or. solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate. in a precipitation reaction, a subclass of exchange reactions, an. Barium Bromide Is A Precipitate.
From physchemcorner.weebly.com
Precipitate Reactions The Science Corner Barium Bromide Is A Precipitate a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: Babr 2 (aq) + so. Sodium chloride,. Barium Bromide Is A Precipitate.
From www.nanochemazone.com
Barium Bromide Beads Powder Low Price 1 Highly pure Nanochemazone Barium Bromide Is A Precipitate in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate.. Barium Bromide Is A Precipitate.
From chemcraft.su
Barium bromate monohydrate, 99 (pure) chemcraft.su Barium Bromide Is A Precipitate watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: Sodium chloride,. Barium Bromide Is A Precipitate.
From www.numerade.com
SOLVED Potassium sulfate solution reacts with barium bromide solution Barium Bromide Is A Precipitate solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate. Babr 2 (aq) + so. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4). Barium Bromide Is A Precipitate.
From www.thoughtco.com
Precipitate Definition and Example in Chemistry Barium Bromide Is A Precipitate watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. . Barium Bromide Is A Precipitate.
From www.pinterest.com
Precipitation Rules.JPG (800×582) Solubility, Chemistry, Precipitation Barium Bromide Is A Precipitate Babr 2 (aq) + so. it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. Sodium chloride, sodium hydroxide, or. a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. in a precipitation reaction, a subclass of exchange. Barium Bromide Is A Precipitate.
From www.youtube.com
Sodium bromide YouTube Barium Bromide Is A Precipitate it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. Sodium chloride, sodium hydroxide, or. Babr 2 (aq) + so. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. a typical precipitation reaction occurs. Barium Bromide Is A Precipitate.
From www.numerade.com
SOLVED Does a solution A solution B precipitate form when A and B are Barium Bromide Is A Precipitate solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate. Babr 2 (aq) + so. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4). Barium Bromide Is A Precipitate.
From www.numerade.com
SOLVED Which aqueous compound below will mix with an aqueous solution Barium Bromide Is A Precipitate in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. Sodium chloride, sodium hydroxide, or. Babr 2 (aq) + so. which solution could be used. Barium Bromide Is A Precipitate.
From www.youtube.com
How to write the equation for Equation for BaBr2 + H2O (Barium bromide Barium Bromide Is A Precipitate it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: Sodium chloride, sodium hydroxide, or. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions. Barium Bromide Is A Precipitate.
From chemcraft.su
Barium bromide dihydrate, 98 (pure) chemcraft.su Barium Bromide Is A Precipitate it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate. Babr 2 (aq) + so. a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with. Barium Bromide Is A Precipitate.
From www.facebook.com
Facebook Barium Bromide Is A Precipitate Sodium chloride, sodium hydroxide, or. it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: . Barium Bromide Is A Precipitate.
From www.youtube.com
How to write the formula for barium bromide YouTube Barium Bromide Is A Precipitate Sodium chloride, sodium hydroxide, or. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Babr 2 (aq) + so. solutions of barium bromide reacts with the sulfate salts. Barium Bromide Is A Precipitate.
From www.chegg.com
Solved Question 15 3 pts What mass (g) of barium bromide is Barium Bromide Is A Precipitate watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: it. Barium Bromide Is A Precipitate.
From brainly.in
balance the equation potassium Bromide + barium iodide gives potassium Barium Bromide Is A Precipitate solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: Babr 2 (aq) + so.. Barium Bromide Is A Precipitate.
From solvedlib.com
14) In the reaction below, precipitate is formed. Com… SolvedLib Barium Bromide Is A Precipitate in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Babr 2 (aq) + so. Sodium chloride, sodium hydroxide, or. a typical precipitation reaction occurs when an aqueous solution. Barium Bromide Is A Precipitate.
From chem.libretexts.org
8.3 Precipitation Reactions Chemistry LibreTexts Barium Bromide Is A Precipitate in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: Sodium chloride, sodium hydroxide,. Barium Bromide Is A Precipitate.
From exogdjght.blob.core.windows.net
Magnesium Bromide Lewis Dot at Allen Matus blog Barium Bromide Is A Precipitate in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. watch this video to learn about precipitation reactions, a type of chemical reaction that produces. Barium Bromide Is A Precipitate.
From www.slideshare.net
Precipitates Barium Bromide Is A Precipitate Sodium chloride, sodium hydroxide, or. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: a typical precipitation reaction occurs when an aqueous solution of barium chloride is mixed with one containing sodium sulfate. the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: solutions. Barium Bromide Is A Precipitate.
From www.pw.live
Barium Bromide Formula, Structure, Properties, Uses Barium Bromide Is A Precipitate it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. Babr 2 (aq) + so. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when. Barium Bromide Is A Precipitate.
From www.procurementresource.com
Barium Bromide Production Cost Analysis Reports 2024 Barium Bromide Is A Precipitate in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. watch this video to learn about precipitation reactions, a type of chemical reaction that produces. Barium Bromide Is A Precipitate.
From lambdageeks.com
Barium Lewis Dot Structure Drawing, Several Compounds and Detailed Barium Bromide Is A Precipitate which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Sodium chloride, sodium hydroxide, or. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. watch this video to learn about precipitation reactions, a type of chemical reaction that. Barium Bromide Is A Precipitate.
From www.youtube.com
How to Write the Net Ionic Equation for CoCl2 + NaOH = Co(OH)2 + NaCl Barium Bromide Is A Precipitate watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate. in a precipitation. Barium Bromide Is A Precipitate.
From www.numerade.com
SOLVED A cesium sulfate solution reacts with barium bromide solution Barium Bromide Is A Precipitate the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates.. Barium Bromide Is A Precipitate.
From www.labdepotinc.com
Barium Bromide Dihydrate Barium Bromide Is A Precipitate Babr 2 (aq) + so. solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate. the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances. Barium Bromide Is A Precipitate.
From www.tessshebaylo.com
Net Ionic Equation For Ammonium Chloride And Water Tessshebaylo Barium Bromide Is A Precipitate solutions of barium bromide reacts with the sulfate salts to produce a solid precipitate of barium sulfate. Sodium chloride, sodium hydroxide, or. in a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. Babr 2 (aq) + so. watch this video to learn about precipitation reactions,. Barium Bromide Is A Precipitate.
From www.chemistrylearner.com
Doublereplacement (Doubledisplacement) Reaction Barium Bromide Is A Precipitate which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Sodium chloride, sodium hydroxide, or. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: Babr. Barium Bromide Is A Precipitate.
From www.pinterest.com
Ammonium bromide NH₄Br Molecular Geometry Hybridization Molecular Barium Bromide Is A Precipitate Babr 2 (aq) + so. it reacts with the sulphate ion of sulpuric acid (h 2 so 4) to give barium sulphate (baso 4) precipitates. the reaction between barium bromide and hydrofluoric acid produces a solid precipitate of barium fluoride: watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid. Barium Bromide Is A Precipitate.