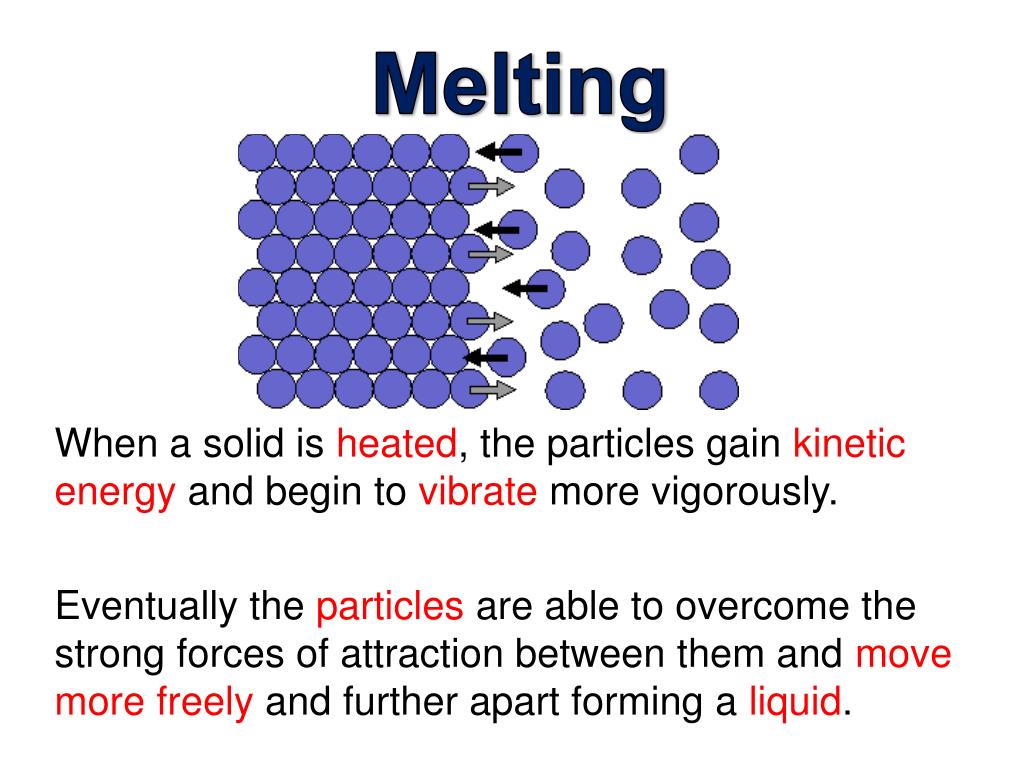
When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules . the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. candles don't burn all by themselves. as a sample of matter is continually cooled, the average kinetic energy of its particles decreases. When a liquid freezes, the reverse. when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. as ice melts into water, kinetic energy is being added to the particles. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the. This causes them to be 'excited' and they break the bonds that hold them. increasing the intermolecular separation increases the internal potential energy component of the internal energy.
from www.slideserve.com
according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the. when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. candles don't burn all by themselves. the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. increasing the intermolecular separation increases the internal potential energy component of the internal energy. This causes them to be 'excited' and they break the bonds that hold them. When a liquid freezes, the reverse. as ice melts into water, kinetic energy is being added to the particles. as a sample of matter is continually cooled, the average kinetic energy of its particles decreases.
PPT The Nature of Matter PowerPoint Presentation, free download ID
When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules as ice melts into water, kinetic energy is being added to the particles. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the. When a liquid freezes, the reverse. as ice melts into water, kinetic energy is being added to the particles. This causes them to be 'excited' and they break the bonds that hold them. candles don't burn all by themselves. the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. as a sample of matter is continually cooled, the average kinetic energy of its particles decreases. increasing the intermolecular separation increases the internal potential energy component of the internal energy.
From physics.stackexchange.com
thermodynamics Could a candle theoretically melt iron? Physics When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. as ice melts into water, kinetic energy is being added to the particles. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the. When a liquid freezes, the reverse.. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From learningschoolinsitoak.z4.web.core.windows.net
Three Stages Of Matter When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules candles don't burn all by themselves. as ice melts into water, kinetic energy is being added to the particles. increasing the intermolecular separation increases the internal potential energy component of the internal energy. When a liquid freezes, the reverse. the heat energy required to convert 1 mole of solid into a liquid at its melting point. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.slideserve.com
PPT 2) The average energy of the molecules in a substance When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules as ice melts into water, kinetic energy is being added to the particles. When a liquid freezes, the reverse. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the. increasing the intermolecular separation increases the internal potential energy component of the internal energy. This causes them to be. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.youtube.com
Time lapse video of candle melting process melting process candle When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the. as a sample of matter is continually cooled, the average kinetic energy of its particles decreases. increasing the intermolecular separation increases the internal potential energy component of the internal energy. This causes them to be 'excited' and they. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules candles don't burn all by themselves. When a liquid freezes, the reverse. increasing the intermolecular separation increases the internal potential energy component of the internal energy. as ice melts into water, kinetic energy is being added to the particles. when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.dreamstime.com
Types of Energy MELTS Scheme Vector Illustration. Labeled Acronym When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules increasing the intermolecular separation increases the internal potential energy component of the internal energy. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the. This causes them to be 'excited' and they break the bonds that hold them. as a sample of matter is continually cooled, the average. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From igcsechemistryrevision.weebly.com
iGCSE CHEMISTRY REVISION HELP Particles & Equations When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules When a liquid freezes, the reverse. increasing the intermolecular separation increases the internal potential energy component of the internal energy. the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. as a sample of matter is continually cooled, the average kinetic energy of its. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From inchemistry.acs.org
Shining a Light on Candles inChemistry When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules as ice melts into water, kinetic energy is being added to the particles. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the. increasing the intermolecular separation increases the internal potential energy component of the internal energy. When a liquid freezes, the reverse. the heat energy required. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules increasing the intermolecular separation increases the internal potential energy component of the internal energy. candles don't burn all by themselves. as a sample of matter is continually cooled, the average kinetic energy of its particles decreases. This causes them to be 'excited' and they break the bonds that hold them. when the imfs are very weak. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.youtube.com
The Science Behind Candle Light How does a candle work??What happens When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules increasing the intermolecular separation increases the internal potential energy component of the internal energy. This causes them to be 'excited' and they break the bonds that hold them. the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. as a sample of matter is. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From devan-bloghuang.blogspot.com
Chemical Properties of Candle Wax When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules as a sample of matter is continually cooled, the average kinetic energy of its particles decreases. as ice melts into water, kinetic energy is being added to the particles. when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. the heat energy required to convert 1 mole. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.pinterest.com.mx
Understanding Physics, Learn Physics, Basic Physics, How To Study When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. as a sample of matter is continually cooled, the average kinetic energy of its particles decreases. When a liquid freezes, the reverse. candles don't burn all by themselves. as ice melts into water,. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.slideserve.com
PPT The Nature of Matter PowerPoint Presentation, free download ID When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules as a sample of matter is continually cooled, the average kinetic energy of its particles decreases. increasing the intermolecular separation increases the internal potential energy component of the internal energy. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the. as ice melts into water, kinetic energy. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules This causes them to be 'excited' and they break the bonds that hold them. as ice melts into water, kinetic energy is being added to the particles. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the. the heat energy required to convert 1 mole of solid into. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From vhmsscience8.weebly.com
Candle Lab VISTA HEIGHTS 8TH GRADE SCIENCE When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules When a liquid freezes, the reverse. when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. increasing the intermolecular separation increases the internal potential energy component of the internal energy. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From studymind.co.uk
Changing State (GCSE Chemistry) Study Mind When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. increasing the intermolecular separation increases the internal potential energy component of the internal energy. as ice melts into water, kinetic energy is being added to the particles. This causes them to be 'excited' and they break the bonds. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From saylordotorg.github.io
The Collision Model of Chemical When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules as a sample of matter is continually cooled, the average kinetic energy of its particles decreases. when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. increasing the intermolecular separation increases the internal potential energy component of the internal energy. When a liquid freezes, the reverse. candles. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.slideserve.com
PPT Average Energy of a Molecule PowerPoint Presentation When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. increasing the intermolecular separation increases the internal potential energy component of the internal energy. candles don't burn all by themselves. When a liquid freezes, the reverse. according to the kinetic molecular theory, the. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.youtube.com
The Average Energy per Molecule Equation for an Ideal Gas IB When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules This causes them to be 'excited' and they break the bonds that hold them. increasing the intermolecular separation increases the internal potential energy component of the internal energy. candles don't burn all by themselves. as ice melts into water, kinetic energy is being added to the particles. according to the kinetic molecular theory, the average kinetic. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From byjus.com
The average internal energy of molecules of a substance is a When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules This causes them to be 'excited' and they break the bonds that hold them. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the. candles don't burn all by themselves. increasing the intermolecular separation increases the internal potential energy component of the internal energy. when the imfs. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.numerade.com
SOLVEDAs a metal such as lead melts, what happens to (a) the average When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. This causes them to be 'excited' and they break the bonds that hold them. the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. as. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From library.achievingthedream.org
Phase Change and Latent Heat Physics When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules as a sample of matter is continually cooled, the average kinetic energy of its particles decreases. increasing the intermolecular separation increases the internal potential energy component of the internal energy. when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. according to the kinetic molecular theory, the. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.youtube.com
Melt the candle or see what happened Candle Melt experiment YouTube When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules candles don't burn all by themselves. When a liquid freezes, the reverse. as ice melts into water, kinetic energy is being added to the particles. when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. the heat energy required to convert 1 mole of solid into a. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.doubtnut.com
What happens to the average energy of the molecules as ice When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules When a liquid freezes, the reverse. the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. This causes them to be 'excited' and they break the bonds that hold them. candles don't burn all by themselves. when the imfs are very weak compared to. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From scozzatobmschematic.z14.web.core.windows.net
What Happens To The Particles When Heated When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. as ice melts into water, kinetic energy is being added to the particles. candles don't burn all by themselves. increasing the intermolecular separation increases the internal potential energy component of the internal energy.. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From slideplayer.com
Unit 6 Heat. ppt download When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. increasing the intermolecular separation increases the internal potential energy component of the internal energy. When a liquid freezes, the reverse. candles don't burn all by themselves. the heat energy required to convert 1 mole of solid into. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.nagwa.com
Question Video Using Figures to Describe What Happens When a Solid When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules This causes them to be 'excited' and they break the bonds that hold them. as ice melts into water, kinetic energy is being added to the particles. When a liquid freezes, the reverse. increasing the intermolecular separation increases the internal potential energy component of the internal energy. according to the kinetic molecular theory, the average kinetic energy. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From id.go-homework.com
Apa yang terjadi pada energi molekulmolekulnya ketika es When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules as ice melts into water, kinetic energy is being added to the particles. the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. as a sample of matter is continually cooled, the average kinetic energy of its particles decreases. when the imfs are. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From studyrocket.co.uk
The Particle Model GCSE Chemistry Science) OCR Revision When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules candles don't burn all by themselves. as a sample of matter is continually cooled, the average kinetic energy of its particles decreases. as ice melts into water, kinetic energy is being added to the particles. when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. the. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From printableawusiloxf.z22.web.core.windows.net
Phase Changes Of Matter Ppt Grade 8 When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules as ice melts into water, kinetic energy is being added to the particles. according to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the. the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. increasing. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.slideserve.com
PPT The Nature of Matter PowerPoint Presentation, free download ID When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules When a liquid freezes, the reverse. increasing the intermolecular separation increases the internal potential energy component of the internal energy. the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. as a sample of matter is continually cooled, the average kinetic energy of its. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From socratic.org
What happens to the energy of its molecules as ice melts into When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules increasing the intermolecular separation increases the internal potential energy component of the internal energy. as ice melts into water, kinetic energy is being added to the particles. This causes them to be 'excited' and they break the bonds that hold them. the heat energy required to convert 1 mole of solid into a liquid at its melting. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From materialdbhutchins.z21.web.core.windows.net
Heat During Phase Change Formula When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules When a liquid freezes, the reverse. as ice melts into water, kinetic energy is being added to the particles. This causes them to be 'excited' and they break the bonds that hold them. when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. according to the kinetic molecular. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From studybrewmaster.z21.web.core.windows.net
What Happens To A Solid When It Melts When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules candles don't burn all by themselves. the heat energy required to convert 1 mole of solid into a liquid at its melting point is called the enthalpy of fusion. This causes them to be 'excited' and they break the bonds that hold them. as a sample of matter is continually cooled, the average kinetic energy of its. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules when the imfs are very weak compared to the ke, the attraction between molecules is minimal, so the molecules. candles don't burn all by themselves. as ice melts into water, kinetic energy is being added to the particles. the heat energy required to convert 1 mole of solid into a liquid at its melting point is. When A Candle Melts What Happens To The Kinetic Energy Of Its Molecules.