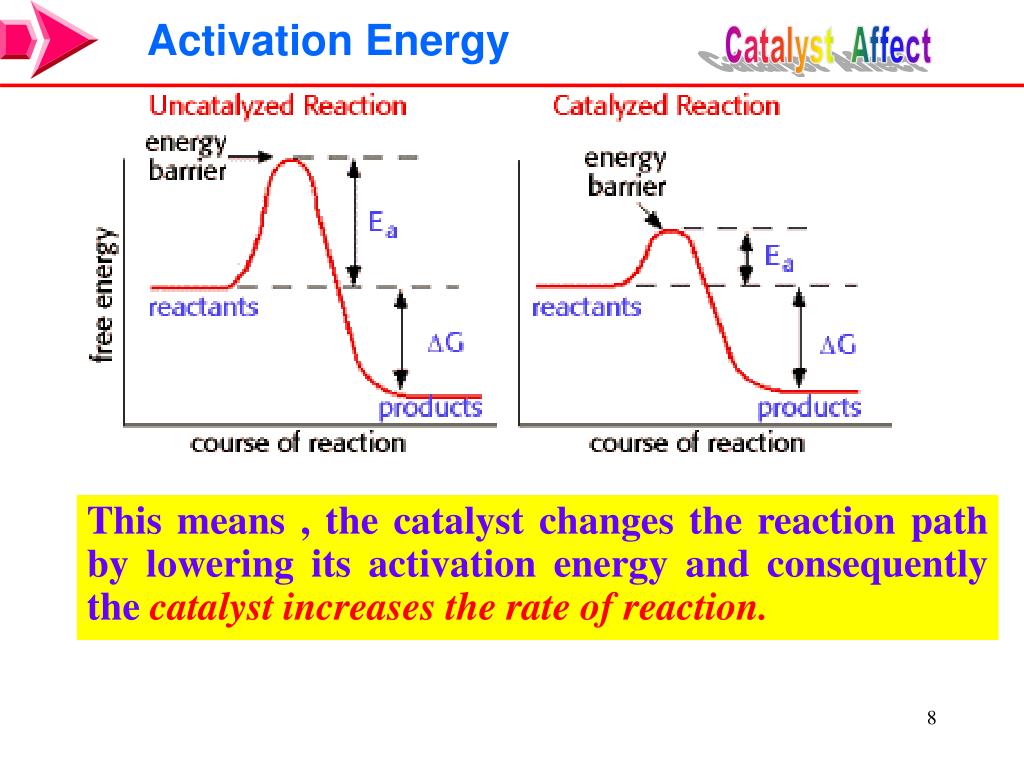
Catalyzed Activation Energy . Learn how catalysts increase the reaction rate and the selectivity of chemical reactions by lowering the activation energy. See examples of homogeneous and heterogeneous catalysis,. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Activation energy is the minimum energy needed to start a chemical reaction. Activation energy is the minimum energy required for a reaction to occur. Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Explore the three major classes of.
from www.slideserve.com
Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. Activation energy is the minimum energy needed to start a chemical reaction. See examples of homogeneous and heterogeneous catalysis,. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. Activation energy is the minimum energy required for a reaction to occur. Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Explore the three major classes of. Learn how catalysts increase the reaction rate and the selectivity of chemical reactions by lowering the activation energy. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it.
PPT Mechanisms of Catalytic Reactions and Characterization of
Catalyzed Activation Energy Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Activation energy is the minimum energy needed to start a chemical reaction. Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Activation energy is the minimum energy required for a reaction to occur. Learn how catalysts increase the reaction rate and the selectivity of chemical reactions by lowering the activation energy. Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. See examples of homogeneous and heterogeneous catalysis,. Explore the three major classes of. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms.
From slideplayer.com
Proteins as Enzymes. ppt download Catalyzed Activation Energy Explore the three major classes of. See examples of homogeneous and heterogeneous catalysis,. Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Learn how catalysts can lower the activation energy. Catalyzed Activation Energy.
From slideplayer.com
Reaction Rates. ppt download Catalyzed Activation Energy Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Activation energy is the minimum energy required for a reaction to occur. Learn how catalysts can lower the activation energy and. Catalyzed Activation Energy.
From www.slideserve.com
PPT Chapter 6 Enzymes PowerPoint Presentation, free download ID1043557 Catalyzed Activation Energy Explore the three major classes of. See examples of homogeneous and heterogeneous catalysis,. Activation energy is the minimum energy required for a reaction to occur. Learn how catalysts increase the reaction rate and the selectivity of chemical reactions by lowering the activation energy. Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to. Catalyzed Activation Energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalyzed Activation Energy Learn how catalysts increase the reaction rate and the selectivity of chemical reactions by lowering the activation energy. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Learn how catalysts. Catalyzed Activation Energy.
From courses.lumenlearning.com
Catalysis Chemistry Catalyzed Activation Energy Activation energy is the minimum energy needed to start a chemical reaction. Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Activation energy is the minimum energy required for a reaction to occur. Explore the three major classes of. Learn how catalysts speed up reactions by lowering the activation energy and providing. Catalyzed Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Catalyzed Activation Energy Activation energy is the minimum energy needed to start a chemical reaction. Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. Learn how activation energy is the minimum energy barrier. Catalyzed Activation Energy.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Catalyzed Activation Energy Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Activation energy is the minimum energy needed to start a chemical reaction. See examples of homogeneous and heterogeneous catalysis,. Learn how catalysts increase the reaction rate and. Catalyzed Activation Energy.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyzed Activation Energy Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. Explore the three major classes of. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Activation energy is the minimum energy required for a. Catalyzed Activation Energy.
From www.meritnation.com
Illustrate graphically the effect of a catalyst on rate of a reaction Catalyzed Activation Energy Explore the three major classes of. Activation energy is the minimum energy needed to start a chemical reaction. Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. Learn how to calculate it, how it. Catalyzed Activation Energy.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.2.4 Activation Energy翰林国际教育 Catalyzed Activation Energy Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. Explore the three major classes of. Activation energy is the minimum energy required for a reaction to occur. Learn how catalysts increase the reaction rate. Catalyzed Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalyzed Activation Energy Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. Explore the three major classes of. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur,. Catalyzed Activation Energy.
From btccasting.weebly.com
Enzymes Lower The Activation Energy Of A Reaction btccasting Catalyzed Activation Energy Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. Explore the three major classes of. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. See examples of homogeneous and heterogeneous catalysis,. Learn how catalysts speed up. Catalyzed Activation Energy.
From quizlet.com
Draw a hypothetical activation energy diagram for an uncatal Quizlet Catalyzed Activation Energy Explore the three major classes of. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts. Catalyzed Activation Energy.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyzed Activation Energy Activation energy is the minimum energy needed to start a chemical reaction. Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Learn how catalysts speed up reactions by. Catalyzed Activation Energy.
From www.slideserve.com
PPT Activation Energy … PowerPoint Presentation, free download ID Catalyzed Activation Energy Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Explore the three major classes of. Learn how catalysts can lower the activation energy and increase the rate of reaction,. Catalyzed Activation Energy.
From wisc.pb.unizin.org
12.7 Catalysis Chemistry Catalyzed Activation Energy Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Explore the three major classes of. See examples of homogeneous and heterogeneous catalysis,. Activation energy is the minimum energy required for a. Catalyzed Activation Energy.
From www.workmanagementsolutions.com.au
An Analogy The Activation Energy and Catalysis in Business Operations Catalyzed Activation Energy Learn how catalysts increase the reaction rate and the selectivity of chemical reactions by lowering the activation energy. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Gas and liquid. Catalyzed Activation Energy.
From www.numerade.com
SOLVED q A + B Energy 2 C + D Progress of the reaction The figure Catalyzed Activation Energy See examples of homogeneous and heterogeneous catalysis,. Activation energy is the minimum energy needed to start a chemical reaction. Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Learn how catalysts increase the. Catalyzed Activation Energy.
From fixportillobadgered.z21.web.core.windows.net
Energy Diagram Activation Energy Catalyzed Activation Energy Explore the three major classes of. Learn how catalysts increase the reaction rate and the selectivity of chemical reactions by lowering the activation energy. Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the.. Catalyzed Activation Energy.
From www.chegg.com
Solved 300+ catalyzed reaction Energy (kJ/mol) uncatalyzed Catalyzed Activation Energy Activation energy is the minimum energy required for a reaction to occur. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. Learn how catalysts increase the reaction. Catalyzed Activation Energy.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Catalyzed Activation Energy Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. Learn how catalysts increase the reaction rate and the selectivity of chemical reactions by lowering the activation energy. Learn how. Catalyzed Activation Energy.
From cheminfo.uz
Kataliz va katalizator haqida ChemInfo.uz Catalyzed Activation Energy Activation energy is the minimum energy required for a reaction to occur. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Learn how catalysts increase the reaction rate and the selectivity of chemical reactions by lowering the activation energy. Learn how to calculate it, how it affects. Catalyzed Activation Energy.
From socratic.org
What are activation energies? Socratic Catalyzed Activation Energy Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Activation energy is the minimum energy needed to start a chemical reaction. Explore the three major classes of. Gas and liquid phase reactions catalyzed by heterogeneous catalysts. Catalyzed Activation Energy.
From www.coursehero.com
[Solved] presents activation energy for the reverse catalyzed reaction Catalyzed Activation Energy Learn how catalysts increase the reaction rate and the selectivity of chemical reactions by lowering the activation energy. Explore the three major classes of. Activation energy is the minimum energy needed to start a chemical reaction. Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Learn how catalysts can lower the activation. Catalyzed Activation Energy.
From www.coursehero.com
[Solved] presents activation energy for the reverse catalyzed reaction Catalyzed Activation Energy Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Learn how catalysts increase the reaction rate and the selectivity of chemical reactions by lowering the activation energy. Activation energy is the minimum energy needed to start a chemical reaction. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of. Catalyzed Activation Energy.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalyzed Activation Energy Activation energy is the minimum energy required for a reaction to occur. Learn how catalysts increase the reaction rate and the selectivity of chemical reactions by lowering the activation energy. Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. Learn how catalysts speed up reactions by lowering the activation energy. Catalyzed Activation Energy.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyzed Activation Energy Activation energy is the minimum energy needed to start a chemical reaction. Explore the three major classes of. Activation energy is the minimum energy required for a reaction to occur. Learn how catalysts increase the reaction rate and the selectivity of chemical reactions by lowering the activation energy. See examples of homogeneous and heterogeneous catalysis,. Learn how catalysts can lower. Catalyzed Activation Energy.
From www.chim.lu
Activation energy and catalysis Catalyzed Activation Energy Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. See examples of homogeneous and heterogeneous catalysis,. Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Learn how to calculate it, how it affects reaction rate, and how enzymes and catalysts lower it. Activation. Catalyzed Activation Energy.
From 2012books.lardbucket.org
Catalysis Catalyzed Activation Energy Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. Explore the three major classes of. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. See examples of homogeneous and heterogeneous catalysis,. Learn how. Catalyzed Activation Energy.
From diagramlisthavens.z21.web.core.windows.net
Reaction Coordinate Diagram With Catalyst Catalyzed Activation Energy Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. See examples of homogeneous and heterogeneous catalysis,. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. Learn how to calculate it, how it affects reaction rate, and. Catalyzed Activation Energy.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyzed Activation Energy Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Activation energy is the minimum energy needed to start a chemical reaction. Activation energy is the minimum energy required for a reaction to occur. Explore the three major classes of. Learn how activation energy is the minimum energy barrier for a chemical reaction to. Catalyzed Activation Energy.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyzed Activation Energy Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. Activation energy is the minimum energy required for a reaction to occur. Activation energy is the minimum energy needed to start a chemical reaction. Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Learn. Catalyzed Activation Energy.
From www.researchgate.net
Reaction heat and activation energy catalyzed by AlCl3/MIL‐53(Al Catalyzed Activation Energy Activation energy is the minimum energy needed to start a chemical reaction. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or liquid. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Learn how to. Catalyzed Activation Energy.
From www.chegg.com
Solved Catalyzed Reaction Rates and Activation Energies A Catalyzed Activation Energy Learn how catalysts can lower the activation energy and increase the rate of reaction, and how to determine the. Explore the three major classes of. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Activation energy is the minimum energy required for a reaction to occur. Activation. Catalyzed Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Catalyzed Activation Energy Learn how catalysts speed up reactions by lowering the activation energy and providing alternative reaction mechanisms. Learn how activation energy is the minimum energy barrier for a chemical reaction to occur, and how catalysts lower the activation energy. Gas and liquid phase reactions catalyzed by heterogeneous catalysts occur on the surface of the catalyst rather than within the gas or. Catalyzed Activation Energy.