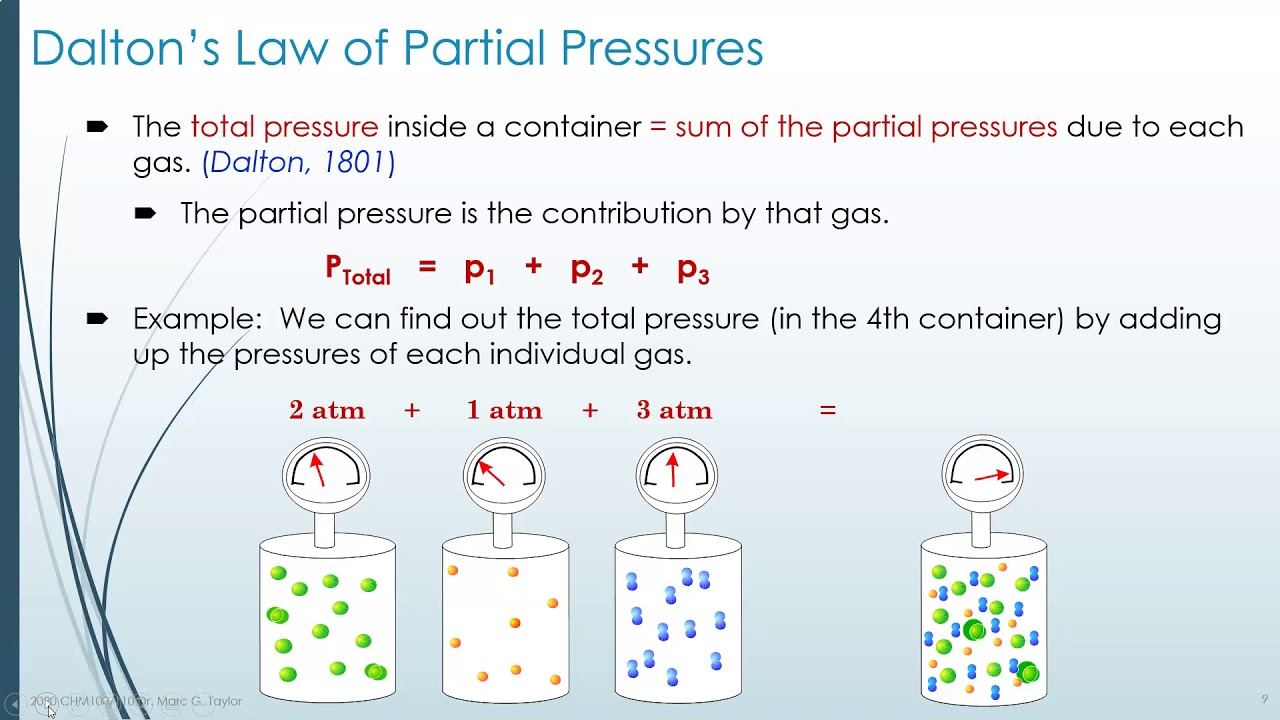
Dalton Law Example . What is dalton’s law of partial pressure. How does it apply to gases. Dalton’s law, also known as dalton’s law of partial pressures, is a fundamental principle in the field of chemistry. Learn its equation along with a few solved problems. Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the partial. Solved examples on dalton’s law of partial pressure. A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. This law states that in a mixture of two or more gases, the total pressure. Dalton's law of partial pressures, or dalton's law, states that the total pressure of a gas in a container is the sum of the partial pressures of the individual gases in the. We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas.
from www.youtube.com
Learn its equation along with a few solved problems. Solved examples on dalton’s law of partial pressure. This law states that in a mixture of two or more gases, the total pressure. We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). Dalton's law of partial pressures, or dalton's law, states that the total pressure of a gas in a container is the sum of the partial pressures of the individual gases in the. How does it apply to gases. Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas. Dalton’s law, also known as dalton’s law of partial pressures, is a fundamental principle in the field of chemistry. What is dalton’s law of partial pressure. Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the partial.
Unit 6b Dalton's Law of Partial Pressures (14min) YouTube
Dalton Law Example Learn its equation along with a few solved problems. Dalton’s law, also known as dalton’s law of partial pressures, is a fundamental principle in the field of chemistry. Learn its equation along with a few solved problems. How does it apply to gases. This law states that in a mixture of two or more gases, the total pressure. Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the partial. A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas. Solved examples on dalton’s law of partial pressure. Dalton's law of partial pressures, or dalton's law, states that the total pressure of a gas in a container is the sum of the partial pressures of the individual gases in the. What is dalton’s law of partial pressure.
From www.slideserve.com
PPT Module C Diffusion PowerPoint Presentation, free download ID Dalton Law Example A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. Dalton’s law, also known as dalton’s law of partial pressures, is a fundamental principle in the field of chemistry. Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of. Dalton Law Example.
From facts.net
19 Surprising Facts About Dalton's Law Of Partial Pressures Dalton Law Example This law states that in a mixture of two or more gases, the total pressure. Learn its equation along with a few solved problems. We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). Dalton's law of partial pressures, or dalton's law, states that the total pressure of a gas in. Dalton Law Example.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressures PowerPoint Presentation, free Dalton Law Example Learn its equation along with a few solved problems. We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each. Dalton Law Example.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressures PowerPoint Presentation, free Dalton Law Example Solved examples on dalton’s law of partial pressure. Learn its equation along with a few solved problems. Dalton's law of partial pressures, or dalton's law, states that the total pressure of a gas in a container is the sum of the partial pressures of the individual gases in the. What is dalton’s law of partial pressure. A mixture of hydrogen. Dalton Law Example.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressure PowerPoint Presentation, free Dalton Law Example Learn its equation along with a few solved problems. Solved examples on dalton’s law of partial pressure. A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is. Dalton Law Example.
From docs.google.com
Dalton's Law of Partial Pressures Worksheet Key.pdf Google Drive Dalton Law Example Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the partial. Dalton's law of partial pressures, or dalton's law, states that the total pressure of a gas in a container is the sum of the partial pressures of the individual gases in the. What. Dalton Law Example.
From www.youtube.com
Daltons Law of Partial Pressures YouTube Dalton Law Example Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas. A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. Solved examples on dalton’s law of. Dalton Law Example.
From byjus.com
what is Dalton's law of partial pressure? Dalton Law Example Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the partial. Dalton's law of partial pressures, or dalton's law, states that the total pressure of a gas in a container is the sum of the partial pressures of the individual gases in the. This. Dalton Law Example.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4326922 Dalton Law Example Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas. Dalton's law of partial pressures, or dalton's law, states that the total pressure of a gas in a container is the sum of the partial pressures of. Dalton Law Example.
From studylib.net
I. Dalton’s Law Dalton Law Example How does it apply to gases. Solved examples on dalton’s law of partial pressure. A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. Dalton’s law, also known as dalton’s law of partial pressures, is a fundamental principle in the field of chemistry. We have just worked out an. Dalton Law Example.
From www.youtube.com
Dalton's Law of Partial Pressure Problems, Mole Fraction, Chemistry Gas Dalton Law Example Dalton's law of partial pressures, or dalton's law, states that the total pressure of a gas in a container is the sum of the partial pressures of the individual gases in the. Solved examples on dalton’s law of partial pressure. What is dalton’s law of partial pressure. Dalton’s law, or the law of partial pressures, states that the total pressure. Dalton Law Example.
From www.slideserve.com
PPT Unit Gas Laws PowerPoint Presentation, free download ID6547905 Dalton Law Example We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. This law states that in a mixture of two or more gases, the total pressure. What is dalton’s law of. Dalton Law Example.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressures PowerPoint Presentation, free Dalton Law Example Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the partial. What is dalton’s law of partial pressure. Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum. Dalton Law Example.
From www.slideserve.com
PPT Properties of Gases & Gas Laws PowerPoint Presentation, free Dalton Law Example Solved examples on dalton’s law of partial pressure. Dalton’s law, also known as dalton’s law of partial pressures, is a fundamental principle in the field of chemistry. We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). This law states that in a mixture of two or more gases, the total. Dalton Law Example.
From www.youtube.com
Chapter 11 17 PROBLEM Dalton's Law of Partial Pressures YouTube Dalton Law Example Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the partial. Dalton's law of partial pressures, or dalton's law, states that the total pressure of a gas in a container is the sum of the partial pressures of the individual gases in the. A. Dalton Law Example.
From www.youtube.com
6.05 Dalton's Law of Partial Pressures YouTube Dalton Law Example A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas. This law states that in a. Dalton Law Example.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressure PowerPoint Presentation, free Dalton Law Example Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas. A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. Dalton’s law, also known as dalton’s. Dalton Law Example.
From www.slideshare.net
Dalton's Law of Partial Pressure Dalton Law Example A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. Solved examples on dalton’s law of partial pressure. Dalton’s law, also known as dalton’s law of partial pressures, is a fundamental principle in the field of chemistry. How does it apply to gases. This law states that in a. Dalton Law Example.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressure PowerPoint Presentation, free Dalton Law Example A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. What is dalton’s law of partial pressure. Dalton’s law, also known as dalton’s law of partial pressures, is a fundamental principle in the field of chemistry. Dalton’s law, or the law of partial pressures, states that the total pressure. Dalton Law Example.
From users.highland.edu
Dalton's Law Dalton Law Example What is dalton’s law of partial pressure. A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. This law states that in a mixture of two or more gases, the total pressure. Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture. Dalton Law Example.
From www.slideserve.com
PPT Daltons Law PowerPoint Presentation, free download ID1790321 Dalton Law Example This law states that in a mixture of two or more gases, the total pressure. Dalton’s law, or the law of partial pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the partial. Solved examples on dalton’s law of partial pressure. Dalton's law of partial pressures, or dalton's law, states that. Dalton Law Example.
From cartoondealer.com
Dalton`s Law, Chemical Physics Example Information Poster With Partial Dalton Law Example Dalton's law of partial pressures, or dalton's law, states that the total pressure of a gas in a container is the sum of the partial pressures of the individual gases in the. We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). Dalton’s law, or the law of partial pressures, states. Dalton Law Example.
From sciencenotes.org
Dalton's Law of Partial Pressure Definition and Examples Dalton Law Example How does it apply to gases. Dalton’s law, also known as dalton’s law of partial pressures, is a fundamental principle in the field of chemistry. This law states that in a mixture of two or more gases, the total pressure. We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). Dalton's. Dalton Law Example.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressure PowerPoint Presentation, free Dalton Law Example What is dalton’s law of partial pressure. A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. Dalton’s law, also known as dalton’s law of partial pressures, is a fundamental principle in the field of chemistry. Dalton’s law, or the law of partial pressures, states that the total pressure. Dalton Law Example.
From www.pinterest.com
Law of Multiple Proportions Dalton's Law Dalton Law Example We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). Learn its equation along with a few solved problems. Dalton’s law, also known as dalton’s law of partial pressures, is a fundamental principle in the field of chemistry. A mixture of hydrogen gas and oxygen gas exerts a total pressure of. Dalton Law Example.
From www.youtube.com
Chemistry 7.6 Dalton's Law of Partial Pressures YouTube Dalton Law Example How does it apply to gases. Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas. What is dalton’s law of partial pressure. This law states that in a mixture of two or more gases, the total. Dalton Law Example.
From www.pinterest.se
Laws of science and medicine Gas laws chemistry, Dalton's law, How to Dalton Law Example What is dalton’s law of partial pressure. We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). Learn its equation along with a few solved problems. How does it apply to gases. A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of. Dalton Law Example.
From www.youtube.com
Dalton's Law of Partial Pressures Explained YouTube Dalton Law Example Learn its equation along with a few solved problems. This law states that in a mixture of two or more gases, the total pressure. Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas. We have just. Dalton Law Example.
From www.worksheetsplanet.com
Dalton's Law Formula + Definition Dalton Law Example Learn its equation along with a few solved problems. This law states that in a mixture of two or more gases, the total pressure. We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). What is dalton’s law of partial pressure. Dalton’s law, or the law of partial pressures, states that. Dalton Law Example.
From www.expii.com
Dalton's Law of Multiple Proportions — Overview & Origin Expii Dalton Law Example Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas. This law states that in a mixture of two or more gases, the total pressure. Learn its equation along with a few solved problems. A mixture of. Dalton Law Example.
From www.thehansindia.com
HR leaders must learn Dalton’s law in chemistry Dalton Law Example We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). Solved examples on dalton’s law of partial pressure. A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. Dalton's law of partial pressures, or dalton's law, states that the total. Dalton Law Example.
From www.youtube.com
Unit 6b Dalton's Law of Partial Pressures (14min) YouTube Dalton Law Example Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas. This law states that in a mixture of two or more gases, the total pressure. What is dalton’s law of partial pressure. Dalton's law of partial pressures,. Dalton Law Example.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation ID352441 Dalton Law Example Dalton’s law, also known as dalton’s law of partial pressures, is a fundamental principle in the field of chemistry. This law states that in a mixture of two or more gases, the total pressure. How does it apply to gases. We have just worked out an example of dalton’s law of partial pressures (named for john dalton, its discoverer). A. Dalton Law Example.
From www.bharatagritech.com
Quiz Worksheet Using Dalton's Law Of Partial Pressures, 52 OFF Dalton Law Example This law states that in a mixture of two or more gases, the total pressure. Dalton’s law, also known as dalton’s law of partial pressures, is a fundamental principle in the field of chemistry. Dalton’s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum. Dalton Law Example.
From www.youtube.com
Dalton's Law of Partial Pressure YouTube Dalton Law Example A mixture of hydrogen gas and oxygen gas exerts a total pressure of 1.5 atm on the walls of its container. Dalton's law of partial pressures, or dalton's law, states that the total pressure of a gas in a container is the sum of the partial pressures of the individual gases in the. Solved examples on dalton’s law of partial. Dalton Law Example.