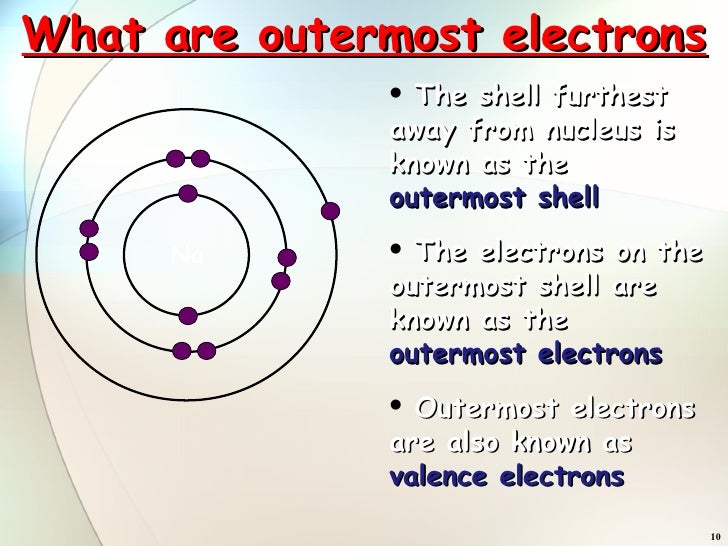
What Is The Electrons Outermost Shell Called . Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. 2.5.3 illustrates the emission of. Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. The valence shell contains the electrons most likely to be. An electron shell is the outside part of an atom. It belongs to a group called the noble gases. When an electron jumps from a higher shell to a lower shell, it emits radiation equal to the energy gap between the initial and the final shell. This means that it will not react with other atoms. The valence shell is the outermost shell of an atom in its neutral state. An atom that has the maximum number of electrons in its outer shell will be stable. Bohr diagrams indicate how many electrons fill each principal shell.
from www.slideshare.net
Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. The valence shell is the outermost shell of an atom in its neutral state. An atom that has the maximum number of electrons in its outer shell will be stable. This means that it will not react with other atoms. When an electron jumps from a higher shell to a lower shell, it emits radiation equal to the energy gap between the initial and the final shell. It belongs to a group called the noble gases. An electron shell is the outside part of an atom. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The valence shell contains the electrons most likely to be.
Structure Of Atoms Part 3
What Is The Electrons Outermost Shell Called The valence shell contains the electrons most likely to be. The valence shell contains the electrons most likely to be. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. This means that it will not react with other atoms. 2.5.3 illustrates the emission of. When an electron jumps from a higher shell to a lower shell, it emits radiation equal to the energy gap between the initial and the final shell. Bohr diagrams indicate how many electrons fill each principal shell. Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. An atom that has the maximum number of electrons in its outer shell will be stable. An electron shell is the outside part of an atom. The valence shell is the outermost shell of an atom in its neutral state. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. It belongs to a group called the noble gases.
From www.nagwa.com
Question Video Identifying the Number of Electrons in the Outermost What Is The Electrons Outermost Shell Called An electron shell is the outside part of an atom. An atom that has the maximum number of electrons in its outer shell will be stable. The valence shell contains the electrons most likely to be. The valence shell is the outermost shell of an atom in its neutral state. Bohr diagrams indicate how many electrons fill each principal shell.. What Is The Electrons Outermost Shell Called.
From www.pinterest.com
valence electrons are electrons on the outermost shell of an atom What Is The Electrons Outermost Shell Called Bohr diagrams indicate how many electrons fill each principal shell. It belongs to a group called the noble gases. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. The valence shell is the outermost shell of an atom in its neutral state. An electron shell is the. What Is The Electrons Outermost Shell Called.
From www.britannica.com
Electron shell Definition & Facts Britannica What Is The Electrons Outermost Shell Called An atom that has the maximum number of electrons in its outer shell will be stable. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. It belongs to a group called the noble gases. Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated. What Is The Electrons Outermost Shell Called.
From philschatz.com
The Building Blocks of Molecules · Concepts of Biology What Is The Electrons Outermost Shell Called This means that it will not react with other atoms. It belongs to a group called the noble gases. 2.5.3 illustrates the emission of. Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. Bohr diagrams indicate how many electrons fill each principal shell. An atom that has the maximum number of. What Is The Electrons Outermost Shell Called.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell What Is The Electrons Outermost Shell Called Bohr diagrams indicate how many electrons fill each principal shell. The valence shell is the outermost shell of an atom in its neutral state. Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence,. What Is The Electrons Outermost Shell Called.
From boisestate.pressbooks.pub
3.4 Electronic Structure of Atoms (Electron Configurations) General What Is The Electrons Outermost Shell Called 2.5.3 illustrates the emission of. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. The valence shell contains the electrons most likely to be. An atom that has the maximum number of electrons in its outer shell will be stable. When an electron jumps from a higher. What Is The Electrons Outermost Shell Called.
From electronicsreference.com
What is Electricity? Electronics Reference What Is The Electrons Outermost Shell Called Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. An electron shell is the outside part of an atom. An atom that has the maximum number of electrons in its outer shell will be stable. The valence shell is defined as the outermost shell of an atom, containing the electrons most. What Is The Electrons Outermost Shell Called.
From sciencenotes.org
Periodic Table Showing Shells What Is The Electrons Outermost Shell Called Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. The valence shell is the outermost shell of an atom in its neutral state. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. An electron shell is the outside. What Is The Electrons Outermost Shell Called.
From brainly.in
an atom of an element has 4 electrons in the outermost M shell. what What Is The Electrons Outermost Shell Called An electron shell is the outside part of an atom. The valence shell contains the electrons most likely to be. 2.5.3 illustrates the emission of. It belongs to a group called the noble gases. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The valence shell is defined as the. What Is The Electrons Outermost Shell Called.
From www.slideshare.net
Structure Of Atoms Part 3 What Is The Electrons Outermost Shell Called When an electron jumps from a higher shell to a lower shell, it emits radiation equal to the energy gap between the initial and the final shell. The valence shell contains the electrons most likely to be. 2.5.3 illustrates the emission of. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to. What Is The Electrons Outermost Shell Called.
From www.allaboutcircuits.com
Quantum Physics Solidstate Device Theory Electronics Textbook What Is The Electrons Outermost Shell Called This means that it will not react with other atoms. Bohr diagrams indicate how many electrons fill each principal shell. When an electron jumps from a higher shell to a lower shell, it emits radiation equal to the energy gap between the initial and the final shell. An electron shell is the outside part of an atom. An atom that. What Is The Electrons Outermost Shell Called.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind What Is The Electrons Outermost Shell Called 2.5.3 illustrates the emission of. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. This means that it will not react with other atoms. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. It belongs to a. What Is The Electrons Outermost Shell Called.
From lessonschoollargest.z14.web.core.windows.net
Electrons In Atoms Explained What Is The Electrons Outermost Shell Called An electron shell is the outside part of an atom. Bohr diagrams indicate how many electrons fill each principal shell. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. This means that it will not react with other atoms. The valence shell is the outermost shell of. What Is The Electrons Outermost Shell Called.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is The Electrons Outermost Shell Called It belongs to a group called the noble gases. Bohr diagrams indicate how many electrons fill each principal shell. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. This means that it will not react with other atoms. When an electron jumps from a higher shell to. What Is The Electrons Outermost Shell Called.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Is The Electrons Outermost Shell Called It belongs to a group called the noble gases. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The valence shell is the outermost shell of an atom in its neutral state. An atom that has the maximum number of electrons in its outer shell will be stable. Bohr diagrams. What Is The Electrons Outermost Shell Called.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table What Is The Electrons Outermost Shell Called It belongs to a group called the noble gases. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. This means that it will not react with other atoms. The valence shell is the outermost shell of an atom in its neutral state. Bohr diagrams indicate how many. What Is The Electrons Outermost Shell Called.
From www.slideserve.com
PPT Chemistry for Bio 9 PowerPoint Presentation, free download ID What Is The Electrons Outermost Shell Called An atom that has the maximum number of electrons in its outer shell will be stable. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. This means that it will not react with other atoms. When an electron jumps from a higher shell to a lower shell,. What Is The Electrons Outermost Shell Called.
From www.britannica.com
Atom Proton, Neutron, Nucleus Britannica What Is The Electrons Outermost Shell Called An atom that has the maximum number of electrons in its outer shell will be stable. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Bohr diagrams indicate how many electrons fill each principal shell. It belongs to a group called the noble gases. This means that it will not. What Is The Electrons Outermost Shell Called.
From icdsc.org
How many electrons are in each shell including 3p orbitals What Is The Electrons Outermost Shell Called The valence shell is the outermost shell of an atom in its neutral state. This means that it will not react with other atoms. The valence shell contains the electrons most likely to be. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. 2.5.3 illustrates the emission of. Arrangement of. What Is The Electrons Outermost Shell Called.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is The Electrons Outermost Shell Called It belongs to a group called the noble gases. The valence shell is the outermost shell of an atom in its neutral state. An atom that has the maximum number of electrons in its outer shell will be stable. Bohr diagrams indicate how many electrons fill each principal shell. When an electron jumps from a higher shell to a lower. What Is The Electrons Outermost Shell Called.
From chem.libretexts.org
9.3 Electron Configurations How Electrons Occupy Orbitals Chemistry What Is The Electrons Outermost Shell Called An atom that has the maximum number of electrons in its outer shell will be stable. Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. This means that it will not react. What Is The Electrons Outermost Shell Called.
From courses.lumenlearning.com
Electrons Biology for Majors I What Is The Electrons Outermost Shell Called This means that it will not react with other atoms. 2.5.3 illustrates the emission of. Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. An electron shell is the outside part of an atom. When an electron jumps from a higher shell to a lower shell, it emits radiation equal to. What Is The Electrons Outermost Shell Called.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science What Is The Electrons Outermost Shell Called Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Bohr diagrams indicate how many electrons fill each principal shell. An electron shell is the outside part of an atom. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in. What Is The Electrons Outermost Shell Called.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is The Electrons Outermost Shell Called The valence shell contains the electrons most likely to be. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Bohr diagrams indicate how many electrons fill each principal shell. It belongs to a group called the noble gases. An atom that has the maximum number of electrons in its outer. What Is The Electrons Outermost Shell Called.
From www.pinterest.com
Valence Electrons• The electrons in the outer most electron shell are What Is The Electrons Outermost Shell Called The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. 2.5.3 illustrates the emission of. Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. An electron shell is the outside part of an atom. When an electron jumps from. What Is The Electrons Outermost Shell Called.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo What Is The Electrons Outermost Shell Called 2.5.3 illustrates the emission of. Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. Bohr diagrams indicate how many electrons fill each principal shell. When an electron jumps from a higher shell to a lower shell, it emits radiation equal to the energy gap between the initial and the final shell.. What Is The Electrons Outermost Shell Called.
From brainly.in
an atom of an element has four electron in the outermost M shell what What Is The Electrons Outermost Shell Called An electron shell is the outside part of an atom. Bohr diagrams indicate how many electrons fill each principal shell. This means that it will not react with other atoms. 2.5.3 illustrates the emission of. An atom that has the maximum number of electrons in its outer shell will be stable. The valence shell is the outermost shell of an. What Is The Electrons Outermost Shell Called.
From stock.adobe.com
Diagram of an oxygen atom with nucleus and inner and outer shells What Is The Electrons Outermost Shell Called This means that it will not react with other atoms. An electron shell is the outside part of an atom. The valence shell contains the electrons most likely to be. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The valence shell is the outermost shell of an atom in. What Is The Electrons Outermost Shell Called.
From commons.wikimedia.org
FilePeriodic table of elements showing electron shells.png What Is The Electrons Outermost Shell Called Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. 2.5.3 illustrates the emission of. When an electron jumps from a higher shell to a lower shell, it emits radiation equal to the energy gap between the initial and the final shell. The valence shell is the outermost shell of an. What Is The Electrons Outermost Shell Called.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free What Is The Electrons Outermost Shell Called Bohr diagrams indicate how many electrons fill each principal shell. Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. It belongs to a group called the noble gases. This means that it will not react with other atoms. The valence shell contains the electrons most likely to be. An atom that. What Is The Electrons Outermost Shell Called.
From www.animalia-life.club
Electron Shell Diagram What Is The Electrons Outermost Shell Called It belongs to a group called the noble gases. This means that it will not react with other atoms. 2.5.3 illustrates the emission of. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. The valence shell contains the electrons most likely to be. When an electron jumps. What Is The Electrons Outermost Shell Called.
From mungfali.com
What Is An Electron Shell What Is The Electrons Outermost Shell Called When an electron jumps from a higher shell to a lower shell, it emits radiation equal to the energy gap between the initial and the final shell. Bohr diagrams indicate how many electrons fill each principal shell. Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. It belongs to a group. What Is The Electrons Outermost Shell Called.
From periodictable.me
Valence+Electrons+The+electrons+on+the+outermost+shell+of+an+atom What Is The Electrons Outermost Shell Called An electron shell is the outside part of an atom. It belongs to a group called the noble gases. 2.5.3 illustrates the emission of. When an electron jumps from a higher shell to a lower shell, it emits radiation equal to the energy gap between the initial and the final shell. The valence shell contains the electrons most likely to. What Is The Electrons Outermost Shell Called.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Is The Electrons Outermost Shell Called When an electron jumps from a higher shell to a lower shell, it emits radiation equal to the energy gap between the initial and the final shell. The valence shell is defined as the outermost shell of an atom, containing the electrons most likely to be involved in chemical. 2.5.3 illustrates the emission of. The valence shell contains the electrons. What Is The Electrons Outermost Shell Called.
From courses.lumenlearning.com
Electrons Biology for Majors I What Is The Electrons Outermost Shell Called It belongs to a group called the noble gases. Arrangement of electron (shell model) is shared under a license and was authored, remixed, and/or curated by libretexts. An atom that has the maximum number of electrons in its outer shell will be stable. An electron shell is the outside part of an atom. The valence shell is the outermost shell. What Is The Electrons Outermost Shell Called.