Excited State Electron Configuration Of Tin . Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. Use linear response theory on top of dft to find the excitation energies. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. This leads to the casida equations, which can be a little complicated to solve. A) write down the electronic configuration of the first. We can simplify the casida equations.
from www.slideserve.com
We can simplify the casida equations. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. A) write down the electronic configuration of the first. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. This leads to the casida equations, which can be a little complicated to solve. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. Use linear response theory on top of dft to find the excitation energies.
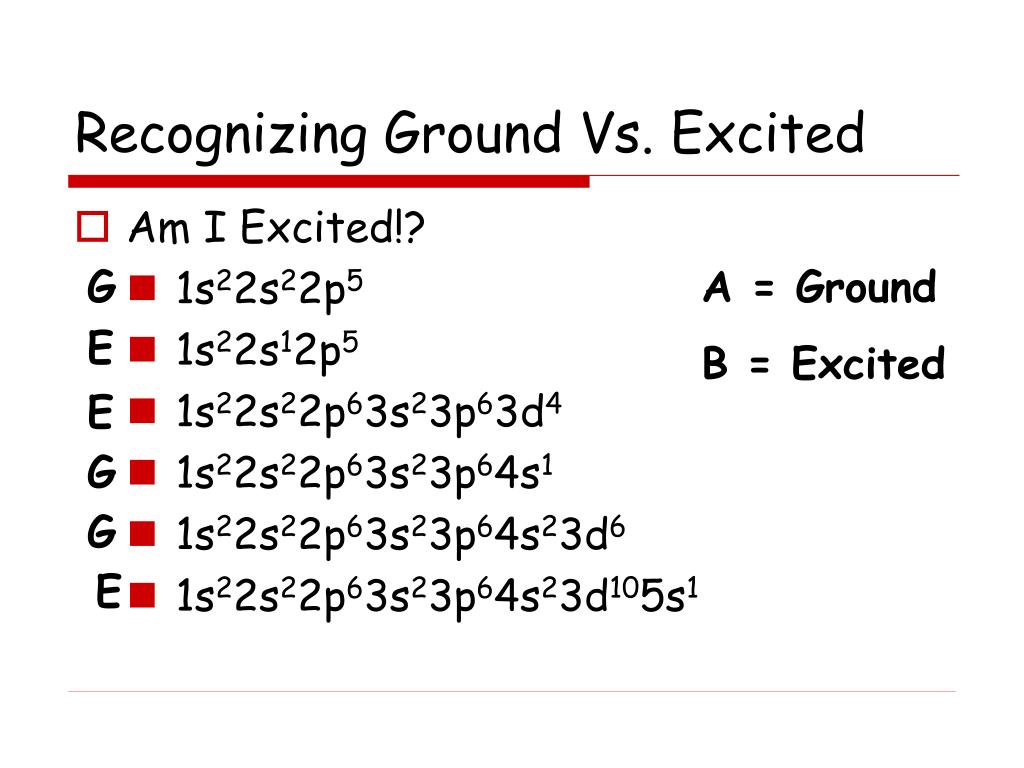
PPT Ground vs. Excited State PowerPoint Presentation, free download ID313264
Excited State Electron Configuration Of Tin Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. Use linear response theory on top of dft to find the excitation energies. A) write down the electronic configuration of the first. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. This leads to the casida equations, which can be a little complicated to solve. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. We can simplify the casida equations.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Excited State Electron Configuration Of Tin An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Use linear response theory on top of dft to find the excitation energies. A) write down the electronic configuration of the first. We can simplify the casida equations. This leads to the casida equations, which can be a little complicated to solve. Tin. Excited State Electron Configuration Of Tin.
From br.pinterest.com
Excited State Easy Science Easy science, Electron configuration, Ap chemistry Excited State Electron Configuration Of Tin Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. A) write down the electronic configuration of the first. We can simplify the casida equations. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. This leads to the casida equations, which can. Excited State Electron Configuration Of Tin.
From slidetodoc.com
3 9 Electron Configurations The electron configuration describes Excited State Electron Configuration Of Tin We can simplify the casida equations. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. A) write down the electronic configuration of the first. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2. Excited State Electron Configuration Of Tin.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Presentation ID1414233 Excited State Electron Configuration Of Tin Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. A) write down the electronic configuration of the first. This leads to the casida equations, which can be a little complicated to solve. Use linear response theory on top of dft to find the excitation energies. An excited state means that. Excited State Electron Configuration Of Tin.
From www.youtube.com
Electron Configuration of Tin Sn Lesson YouTube Excited State Electron Configuration Of Tin A) write down the electronic configuration of the first. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Use linear response theory on top of dft to find the excitation energies. This leads to the casida equations, which can be a little complicated to solve. We can simplify the casida equations. Tin. Excited State Electron Configuration Of Tin.
From www.schoolmykids.com
Tin (Sn) Element Information, Facts, Properties, Uses Periodic Table of the Elements Excited State Electron Configuration Of Tin A) write down the electronic configuration of the first. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. This leads to the casida equations, which can be a little complicated to solve.. Excited State Electron Configuration Of Tin.
From www.researchgate.net
Electronic configurations energy levels of the ground state 3 O 2... Download Scientific Diagram Excited State Electron Configuration Of Tin Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Use linear response theory on top of dft to find the excitation energies. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. We can simplify the casida equations. A) write down the electronic. Excited State Electron Configuration Of Tin.
From valenceelectrons.com
Tin(Sn) electron configuration and orbital diagram Excited State Electron Configuration Of Tin We can simplify the casida equations. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Use linear response theory on top of dft to find the excitation energies. This leads to the casida equations, which can be a little complicated to solve. Tin has a ground state electron configuration of. Excited State Electron Configuration Of Tin.
From www.slideshare.net
Atomic concepts Excited State Electron Configuration Of Tin Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. A) write down the electronic configuration of the first. Use linear response theory on top of dft to find the excitation energies. This leads to the casida equations, which can be a. Excited State Electron Configuration Of Tin.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Excited State Electron Configuration Of Tin Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. This leads to the casida equations, which can be a little complicated to solve. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Use linear response theory on top of dft to find the excitation energies. An excited state means that. Excited State Electron Configuration Of Tin.
From www.slideserve.com
PPT Ground vs. Excited State PowerPoint Presentation, free download ID313264 Excited State Electron Configuration Of Tin We can simplify the casida equations. A) write down the electronic configuration of the first. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Tin has a ground state electron configuration of 1s. Excited State Electron Configuration Of Tin.
From valenceelectrons.com
Tin(Sn) electron configuration and orbital diagram Excited State Electron Configuration Of Tin Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. Use linear response theory on top of dft to find the excitation energies. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration.. Excited State Electron Configuration Of Tin.
From slidetodoc.com
Aim What happens when electrons get excited Do Excited State Electron Configuration Of Tin Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. Use linear response theory on top of dft to find the excitation energies. A) write down the electronic configuration of the first. This leads to the casida equations, which can be a little complicated to solve. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s. Excited State Electron Configuration Of Tin.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Excited State Electron Configuration Of Tin This leads to the casida equations, which can be a little complicated to solve. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Use linear response theory on top of dft to find the excitation energies. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. We can simplify the casida. Excited State Electron Configuration Of Tin.
From www.newtondesk.com
Tin Sn (Element 50) of Periodic Table Periodic Table FlashCards Excited State Electron Configuration Of Tin A) write down the electronic configuration of the first. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. We can simplify the casida equations. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e.. Excited State Electron Configuration Of Tin.
From www.chegg.com
Solved Question 2 What is the excited state electron Excited State Electron Configuration Of Tin Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. Use linear response theory on top of dft to find the excitation energies. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. This leads to the casida equations, which can be a. Excited State Electron Configuration Of Tin.
From www.youtube.com
Sn^2+,Sn^4+ and Sn (Tin and Tin Ions) Electron ConfigurationCrash Course Chemistry YouTube Excited State Electron Configuration Of Tin Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Use linear response theory on top of dft to find the excitation energies.. Excited State Electron Configuration Of Tin.
From www.pinterest.com
Electron Configuration Chart for the Elements Chart, Chemistry and Organic chemistry Excited State Electron Configuration Of Tin An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Use linear response theory on top of dft to find the excitation energies. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. A) write down the electronic configuration of the first. Tin has. Excited State Electron Configuration Of Tin.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Presentation ID1414233 Excited State Electron Configuration Of Tin Use linear response theory on top of dft to find the excitation energies. We can simplify the casida equations. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Tin has a ground state. Excited State Electron Configuration Of Tin.
From www.coursehero.com
[Solved] Identify the neutral element represented by this excited‑state... Course Hero Excited State Electron Configuration Of Tin This leads to the casida equations, which can be a little complicated to solve. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Excited State Electron Configuration Of Tin.
From material-properties.org
Tin Protons Neutrons Electrons Electron Configuration Excited State Electron Configuration Of Tin This leads to the casida equations, which can be a little complicated to solve. A) write down the electronic configuration of the first. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. We can simplify the casida equations. Use linear response theory on top of dft to find the excitation energies. Tin. Excited State Electron Configuration Of Tin.
From www.numerade.com
SOLVED The electron configuration of 1s22s22p63s23p64s13d8 is an excited state electron Excited State Electron Configuration Of Tin Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. A) write down the electronic configuration of the first. This leads to the casida equations, which can be a little complicated to solve. We can simplify the casida equations. An excited state means that (typically) the valence electron has moved from. Excited State Electron Configuration Of Tin.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Presentation ID1414233 Excited State Electron Configuration Of Tin A) write down the electronic configuration of the first. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. This leads to the casida equations, which can be a little complicated to solve. We can simplify the casida equations. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Excited State Electron Configuration Of Tin.
From www.slideserve.com
PPT Ground vs. Excited State PowerPoint Presentation, free download ID313264 Excited State Electron Configuration Of Tin Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. We can simplify the casida equations. A) write down the electronic configuration of. Excited State Electron Configuration Of Tin.
From www.webelements.com
Elements Periodic Table » Tin » properties of free atoms Excited State Electron Configuration Of Tin Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. An excited state means. Excited State Electron Configuration Of Tin.
From aurorecollette.blogspot.com
orbital diagram of tin AuroreCollette Excited State Electron Configuration Of Tin We can simplify the casida equations. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. This leads to the casida equations, which can be a little complicated to solve. A) write down the electronic configuration of the first. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Find the atomic number of. Excited State Electron Configuration Of Tin.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Excited State Electron Configuration Of Tin We can simplify the casida equations. A) write down the electronic configuration of the first. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. Use linear response theory on top of dft. Excited State Electron Configuration Of Tin.
From www.britannica.com
Tin Definition, Properties, Uses, & Facts Britannica Excited State Electron Configuration Of Tin Use linear response theory on top of dft to find the excitation energies. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. A) write down the electronic configuration of the first. This leads to the casida equations, which can be a. Excited State Electron Configuration Of Tin.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Excited State Electron Configuration Of Tin Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. This leads to the casida equations, which can be a little complicated to solve. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Find. Excited State Electron Configuration Of Tin.
From www.youtube.com
Ground State, Excited State, or Impossible Electron Configurations YouTube Excited State Electron Configuration Of Tin We can simplify the casida equations. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. A) write down the electronic configuration of the first. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. This leads to the casida equations, which can. Excited State Electron Configuration Of Tin.
From www.chegg.com
Solved 5 What is the electron configuration for tin (Sn)? (4 Excited State Electron Configuration Of Tin A) write down the electronic configuration of the first. Use linear response theory on top of dft to find the excitation energies. This leads to the casida equations, which can be a little complicated to solve. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. Tin has a ground state electron configuration. Excited State Electron Configuration Of Tin.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Excited State Electron Configuration Of Tin This leads to the casida equations, which can be a little complicated to solve. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. An excited state means that (typically) the valence electron has moved from its ground state orbital (i.e. We can simplify the casida equations. Find the atomic number of nitrogen (7) and use this electron configuration calculator to. Excited State Electron Configuration Of Tin.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Excited State Electron Configuration Of Tin Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. A) write down the electronic configuration of the first. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. We can simplify the casida equations. An excited state means that (typically) the valence. Excited State Electron Configuration Of Tin.
From www.chegg.com
Solved Which of the following represents an excited state Excited State Electron Configuration Of Tin This leads to the casida equations, which can be a little complicated to solve. Use linear response theory on top of dft to find the excitation energies. Tin has a ground state electron configuration of ##[kr]4d^{10}5s^25p^2##. A) write down the electronic configuration of the first. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get. Excited State Electron Configuration Of Tin.
From www.vectorstock.com
Symbol and electron diagram for tin Royalty Free Vector Excited State Electron Configuration Of Tin A) write down the electronic configuration of the first. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10. Find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. This leads to the. Excited State Electron Configuration Of Tin.