Vinegar Zinc Plating . I decided to use white vinegar as i had. Vinegar is a weak acid and dissolves the zinc. The process involves electroplating, running a current of electricity through a. Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. It was recommended by several people to use vinegar or muriatic acid to strip the zinc off. This generates zinc ions in the vinegar. Zinc electroplating allows you to coat metals with a physical zinc layer in order to prevent rust from reaching the metal surface underneath. I never tried it but heard that. Electroplating household metals is the process of coating them with a different metal, and you can easily do it at home. I have used vinegar a few times but for only zinc plated fasteners. Galvanized is probably too thick.
from www.surtecphils.com
It was recommended by several people to use vinegar or muriatic acid to strip the zinc off. For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. This generates zinc ions in the vinegar. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. Galvanized is probably too thick. Zinc electroplating allows you to coat metals with a physical zinc layer in order to prevent rust from reaching the metal surface underneath. I decided to use white vinegar as i had. The process involves electroplating, running a current of electricity through a. I never tried it but heard that. Vinegar is a weak acid and dissolves the zinc.
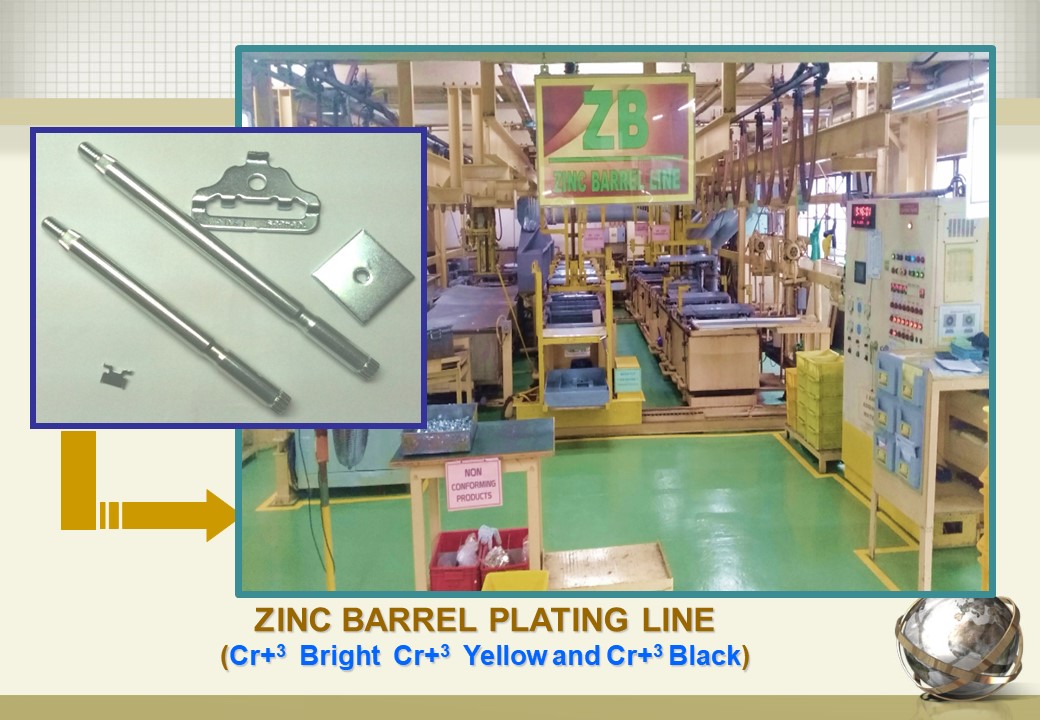
Zinc Barrel Plating Line Surtec philippines, Inc.
Vinegar Zinc Plating Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. The process involves electroplating, running a current of electricity through a. It was recommended by several people to use vinegar or muriatic acid to strip the zinc off. I never tried it but heard that. Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. I have used vinegar a few times but for only zinc plated fasteners. Electroplating household metals is the process of coating them with a different metal, and you can easily do it at home. Vinegar is a weak acid and dissolves the zinc. Galvanized is probably too thick. I decided to use white vinegar as i had. For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. This generates zinc ions in the vinegar. Zinc electroplating allows you to coat metals with a physical zinc layer in order to prevent rust from reaching the metal surface underneath.
From www.ashevillemetalfinishing.com
Zinc Plating Asheville Metal Finishing Asheville, NC Vinegar Zinc Plating I have used vinegar a few times but for only zinc plated fasteners. Electroplating household metals is the process of coating them with a different metal, and you can easily do it at home. This generates zinc ions in the vinegar. I never tried it but heard that. Electrogalvanizing is a process in which a layer of zinc is bonded. Vinegar Zinc Plating.
From www.flickr.com
electroplating solution vinegar, sugar, epsom salt, zinc Flickr Vinegar Zinc Plating Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. This generates zinc ions in the vinegar. Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. I decided to use white vinegar as i had. Galvanized is probably too thick. I never. Vinegar Zinc Plating.
From www.instructables.com
Easy Electroplating 7 Steps Instructables Vinegar Zinc Plating Electroplating household metals is the process of coating them with a different metal, and you can easily do it at home. Vinegar is a weak acid and dissolves the zinc. The process involves electroplating, running a current of electricity through a. I have used vinegar a few times but for only zinc plated fasteners. This generates zinc ions in the. Vinegar Zinc Plating.
From howtormeov.blogspot.com
Will Vinegar Remove Zinc Plating HOWTORMEOV Vinegar Zinc Plating Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. Electroplating household metals is the process of coating them with a different metal, and you can easily do it at home. Galvanized is. Vinegar Zinc Plating.
From paramountmetalfinishing.com
Zinc Plating Benefits ASTM B633 Paramount Metal Finishing Vinegar Zinc Plating Zinc electroplating allows you to coat metals with a physical zinc layer in order to prevent rust from reaching the metal surface underneath. I have used vinegar a few times but for only zinc plated fasteners. Galvanized is probably too thick. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. The process. Vinegar Zinc Plating.
From www.surtecphils.com
Zinc Barrel Plating Line Surtec philippines, Inc. Vinegar Zinc Plating Zinc electroplating allows you to coat metals with a physical zinc layer in order to prevent rust from reaching the metal surface underneath. Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. For electroplating to occur the plating solution must contain metal ions of the metal you want to. Vinegar Zinc Plating.
From mechanicalfinishersinc.blogspot.com
The Different Steps Involved In The Zinc Plating Process Vinegar Zinc Plating I decided to use white vinegar as i had. Galvanized is probably too thick. This generates zinc ions in the vinegar. For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. I never tried it but heard that. Electroplating household metals is the process of coating them with a different metal, and. Vinegar Zinc Plating.
From www.mftech.com
Zinc Plating Services Metal Finishing Tech Vinegar Zinc Plating For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. Zinc electroplating allows you to coat metals with a physical zinc layer in order to prevent rust from reaching the metal surface underneath. Vinegar is a weak acid and dissolves the zinc. I never tried it but heard that. Allow the zinc. Vinegar Zinc Plating.
From www.pinterest.com
Did you know? Zinc plated hardware can quickly get the rustic look Vinegar Zinc Plating Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. Vinegar is a weak acid and dissolves the zinc. This generates zinc ions in the vinegar. Electroplating household metals is the process of coating them with a different metal, and you can easily do it at home. I never tried. Vinegar Zinc Plating.
From www.youtube.com
Zinc + Vinegar Reaction Experiment YouTube Vinegar Zinc Plating Vinegar is a weak acid and dissolves the zinc. I have used vinegar a few times but for only zinc plated fasteners. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. The process involves electroplating, running a current of electricity through a. Galvanized is probably too thick. Electrogalvanizing is a process in. Vinegar Zinc Plating.
From www.sarcoatings.com
Zinc Plating Zinc Plating Service Sar Coatings Vinegar Zinc Plating For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. I have used vinegar a few times but for only zinc plated fasteners. It was recommended by several people to use vinegar or muriatic acid to strip the zinc off. This generates zinc ions in the vinegar. I decided to use white. Vinegar Zinc Plating.
From sun-glo.com
Zinc Rack/Barrel Plating Zinc Electroplating Services Sun Glo Vinegar Zinc Plating Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. Electroplating household metals is the process of coating them with a different metal, and you can easily do it at home. It was recommended by. Vinegar Zinc Plating.
From www.youtube.com
How to zinc plating rust damage motorcycle parts⚗️😱🌠 YouTube Vinegar Zinc Plating Electroplating household metals is the process of coating them with a different metal, and you can easily do it at home. Galvanized is probably too thick. It was recommended by several people to use vinegar or muriatic acid to strip the zinc off. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating.. Vinegar Zinc Plating.
From blog.thepipingmart.com
Zinc Iron Plating Applications and Process Vinegar Zinc Plating I have used vinegar a few times but for only zinc plated fasteners. The process involves electroplating, running a current of electricity through a. Zinc electroplating allows you to coat metals with a physical zinc layer in order to prevent rust from reaching the metal surface underneath. Galvanized is probably too thick. It was recommended by several people to use. Vinegar Zinc Plating.
From www.youtube.com
Alkaline Zinc Plating (Noncyanide) II Electroplating Process II🔥 Vinegar Zinc Plating The process involves electroplating, running a current of electricity through a. Galvanized is probably too thick. This generates zinc ions in the vinegar. Zinc electroplating allows you to coat metals with a physical zinc layer in order to prevent rust from reaching the metal surface underneath. I have used vinegar a few times but for only zinc plated fasteners. For. Vinegar Zinc Plating.
From www.youtube.com
How to Zinc Plate Bolts and Hardware DIY Zinc Plating Tutorial YouTube Vinegar Zinc Plating Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. It was recommended by several people to use vinegar or muriatic acid to strip the zinc off. Zinc electroplating allows you to coat metals with a physical zinc layer in order to prevent rust from reaching the metal surface underneath. Electroplating household metals. Vinegar Zinc Plating.
From blog.thepipingmart.com
How to Remove Nickel Plating with Vinegar Vinegar Zinc Plating The process involves electroplating, running a current of electricity through a. Galvanized is probably too thick. It was recommended by several people to use vinegar or muriatic acid to strip the zinc off. I never tried it but heard that. I decided to use white vinegar as i had. Zinc electroplating allows you to coat metals with a physical zinc. Vinegar Zinc Plating.
From www.metalcheminc.com
Zinc Plating Services ASTM B633 Metal Chem Inc. Vinegar Zinc Plating For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. Galvanized is probably too thick. I decided to use white vinegar as i had. It was recommended by several people to use vinegar or muriatic acid to strip the zinc off. Vinegar is a weak acid and dissolves the zinc. Allow the. Vinegar Zinc Plating.
From sendcutsend.com
The Benefits of Zinc Plating in Laser Cutting SendCutSend Vinegar Zinc Plating I decided to use white vinegar as i had. Zinc electroplating allows you to coat metals with a physical zinc layer in order to prevent rust from reaching the metal surface underneath. Vinegar is a weak acid and dissolves the zinc. I never tried it but heard that. It was recommended by several people to use vinegar or muriatic acid. Vinegar Zinc Plating.
From twbfinishing.co.uk
Zinc Plating TWB Finishing Vinegar Zinc Plating The process involves electroplating, running a current of electricity through a. This generates zinc ions in the vinegar. I decided to use white vinegar as i had. I never tried it but heard that. Zinc electroplating allows you to coat metals with a physical zinc layer in order to prevent rust from reaching the metal surface underneath. Allow the zinc. Vinegar Zinc Plating.
From www.ecwilliams.co.uk
Zinc Plating EC Williams Zinc Nickel Plating Services Vinegar Zinc Plating The process involves electroplating, running a current of electricity through a. Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. I decided to use white vinegar as i had. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. For electroplating to. Vinegar Zinc Plating.
From www.sarcoatings.com
Understand the Electroplating with Zinc Plating Barrels Vinegar Zinc Plating Electroplating household metals is the process of coating them with a different metal, and you can easily do it at home. For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. It was recommended by several people to use vinegar or muriatic acid to strip the zinc off. Galvanized is probably too. Vinegar Zinc Plating.
From blog.thepipingmart.com
Advantages and Disadvantages of Zinc Plating Vinegar Zinc Plating Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. I decided to use white vinegar as i had. For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. Galvanized is probably too thick. Electroplating household metals is the process of coating. Vinegar Zinc Plating.
From giosmdpyc.blob.core.windows.net
How To Zinc Plating at Terri Cohen blog Vinegar Zinc Plating Vinegar is a weak acid and dissolves the zinc. Zinc electroplating allows you to coat metals with a physical zinc layer in order to prevent rust from reaching the metal surface underneath. Galvanized is probably too thick. Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. I never tried. Vinegar Zinc Plating.
From www.mgonitzke.net
Zinc Electroplating Vinegar Zinc Plating I never tried it but heard that. Zinc electroplating allows you to coat metals with a physical zinc layer in order to prevent rust from reaching the metal surface underneath. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. The process involves electroplating, running a current of electricity through a. This generates. Vinegar Zinc Plating.
From ro.pinterest.com
How to Age Hardware New screws soaked overnight in vinegar to remove Vinegar Zinc Plating The process involves electroplating, running a current of electricity through a. It was recommended by several people to use vinegar or muriatic acid to strip the zinc off. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. I decided to use white vinegar as i had. Vinegar is a weak acid and. Vinegar Zinc Plating.
From www.youtube.com
Diy Electroplating 01 Zinc Plating with Vinegar Galvanizing Solution Vinegar Zinc Plating This generates zinc ions in the vinegar. I never tried it but heard that. I decided to use white vinegar as i had. For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. I have used vinegar a few times but for only zinc plated fasteners. It was recommended by several people. Vinegar Zinc Plating.
From www.slideserve.com
PPT Decoding the Diversity of Zinc Plating Types of Zinc Plating Vinegar Zinc Plating I never tried it but heard that. Vinegar is a weak acid and dissolves the zinc. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. Electroplating household metals is the process of coating them with a different metal, and you can easily do it at home. This generates zinc ions in the. Vinegar Zinc Plating.
From sharp-industries.in
Zinc Electroplating Sharp Industries Vinegar Zinc Plating Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. I never tried it but heard that. The process involves electroplating, running a current of electricity through a. This generates zinc ions in the vinegar. It was recommended by several people to use vinegar or muriatic acid to strip the. Vinegar Zinc Plating.
From howtormeov.blogspot.com
Will Vinegar Remove Zinc Plating HOWTORMEOV Vinegar Zinc Plating I have used vinegar a few times but for only zinc plated fasteners. I never tried it but heard that. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. Electroplating household metals. Vinegar Zinc Plating.
From www.sarcoatings.com
Zinc Plating Zinc Plating Service Sar Coatings Vinegar Zinc Plating This generates zinc ions in the vinegar. Galvanized is probably too thick. I have used vinegar a few times but for only zinc plated fasteners. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. I never tried it but heard that. It was recommended by several people to use vinegar or muriatic. Vinegar Zinc Plating.
From www.youtube.com
Acid Zinc plating Process YouTube Vinegar Zinc Plating Galvanized is probably too thick. Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. Vinegar is a weak acid and dissolves the zinc. It was recommended by several people to use vinegar or muriatic acid to strip the zinc off. I have used vinegar a few times but for. Vinegar Zinc Plating.
From www.slideserve.com
PPT Decoding the Diversity of Zinc Plating Types of Zinc Plating Vinegar Zinc Plating For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. Galvanized is probably too thick. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. I decided to use white vinegar as i had. The process involves electroplating, running a current of electricity through a.. Vinegar Zinc Plating.
From www.pinterest.com
DIY Zinc Plating Electroplating electrolysis Electroplating diy, Diy Vinegar Zinc Plating Electroplating household metals is the process of coating them with a different metal, and you can easily do it at home. I decided to use white vinegar as i had. I have used vinegar a few times but for only zinc plated fasteners. Galvanized is probably too thick. The process involves electroplating, running a current of electricity through a. Vinegar. Vinegar Zinc Plating.
From twbfinishing.co.uk
Zinc Plating TWB Finishing Vinegar Zinc Plating I decided to use white vinegar as i had. I never tried it but heard that. Electroplating household metals is the process of coating them with a different metal, and you can easily do it at home. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. I have used vinegar a few. Vinegar Zinc Plating.