What Is An Equilibrium Position . A number that describes how far forward. A particular set of concentrations of reactant and product species. A reversible reaction is one which can be made to go. The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. Identify the physical conditions of static equilibrium. Explain how the conditions for equilibrium. This is essentially what happens if one of the products is removed as soon as it is. The position of equilibrium moves to the left. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means.
from www.javierparra.net
A particular set of concentrations of reactant and product species. Explain how the conditions for equilibrium. A reversible reaction is one which can be made to go. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. A number that describes how far forward. Identify the physical conditions of static equilibrium. The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. This is essentially what happens if one of the products is removed as soon as it is. The position of equilibrium moves to the left.
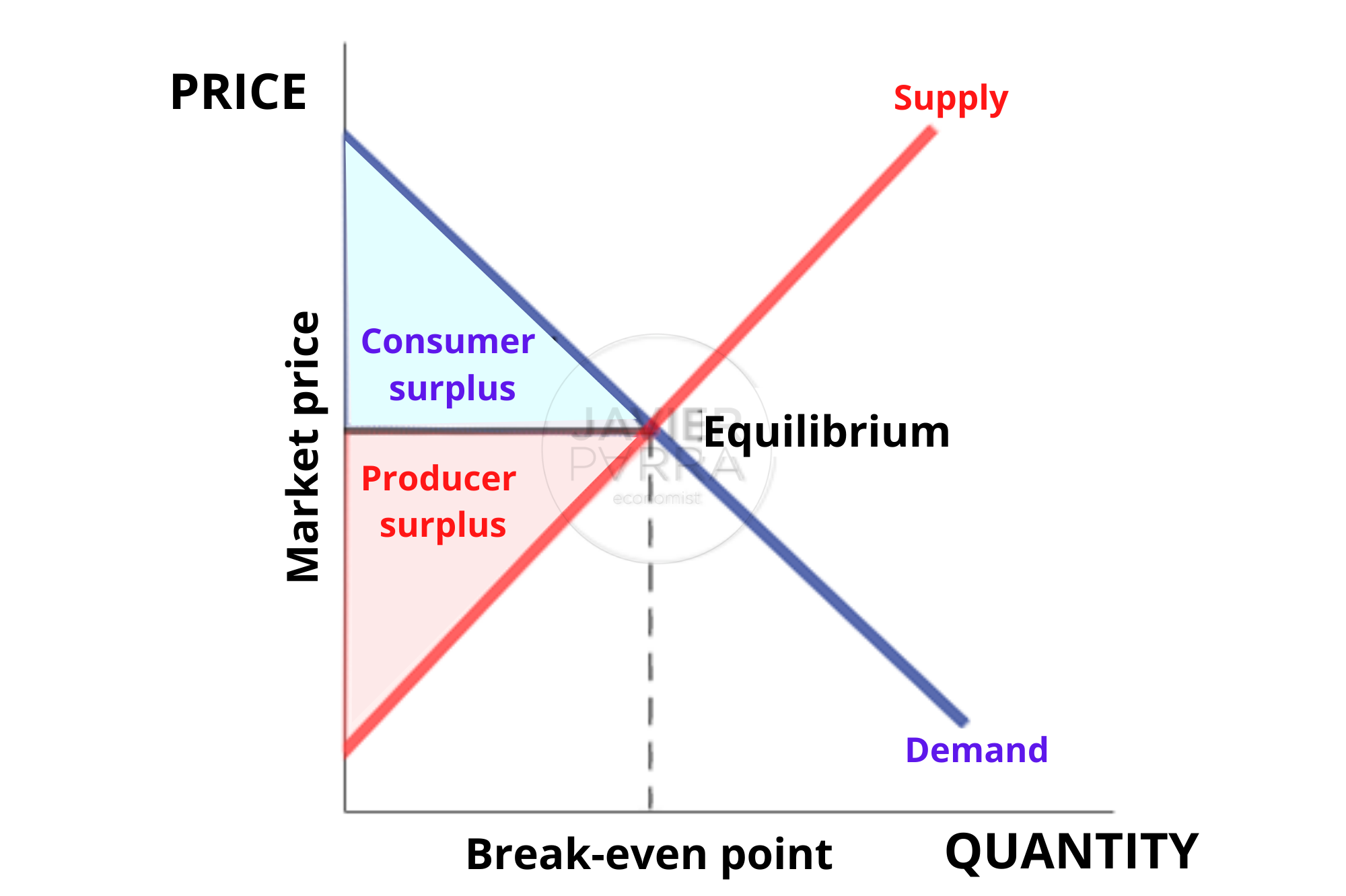
Contents, Economics General equilibrium theory
What Is An Equilibrium Position Identify the physical conditions of static equilibrium. Identify the physical conditions of static equilibrium. Explain how the conditions for equilibrium. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. A reversible reaction is one which can be made to go. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. The position of equilibrium moves to the left. A particular set of concentrations of reactant and product species. A number that describes how far forward. This is essentially what happens if one of the products is removed as soon as it is. The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID3920133 What Is An Equilibrium Position The position of equilibrium moves to the left. A reversible reaction is one which can be made to go. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. A particular set of concentrations of reactant and product species. Identify the physical conditions of static equilibrium. A number that describes how far. What Is An Equilibrium Position.
From www.slideserve.com
PPT Chemical Systems and Equilibrium PowerPoint Presentation, free What Is An Equilibrium Position The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. A number that describes how far forward. This is essentially what happens if one of the products is removed as soon as it is. The position of equilibrium moves to the left. Identify the physical conditions of static equilibrium.. What Is An Equilibrium Position.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID307397 What Is An Equilibrium Position This is essentially what happens if one of the products is removed as soon as it is. Identify the physical conditions of static equilibrium. A number that describes how far forward. A reversible reaction is one which can be made to go. The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium.. What Is An Equilibrium Position.
From www.youtube.com
What is a Position of Equilibrium? YouTube What Is An Equilibrium Position A number that describes how far forward. Explain how the conditions for equilibrium. A particular set of concentrations of reactant and product species. Identify the physical conditions of static equilibrium. A reversible reaction is one which can be made to go. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium. What Is An Equilibrium Position.
From www.slideserve.com
PPT Equilibrium Constant PowerPoint Presentation, free download ID What Is An Equilibrium Position A number that describes how far forward. A reversible reaction is one which can be made to go. The position of equilibrium moves to the left. Explain how the conditions for equilibrium. A particular set of concentrations of reactant and product species. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how. What Is An Equilibrium Position.
From www.slideshare.net
WAVES What Is An Equilibrium Position This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. Explain how the conditions for equilibrium. Identify the physical conditions of static equilibrium. A reversible reaction is one which can be made to go. A number that describes how far forward. The position of equilibrium is a property of the particular reversible. What Is An Equilibrium Position.
From www.researchgate.net
Linear zone around equilibrium position (a) and representation of the What Is An Equilibrium Position This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. A number that describes how far forward. The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium. A reversible reaction is one which can be made to go. This is essentially what happens if. What Is An Equilibrium Position.
From www.grc.nasa.gov
Equilibrium of Three Forces What Is An Equilibrium Position The position of equilibrium moves to the left. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. This is essentially what happens if one of the products is removed as soon as it is. Explain how the conditions for equilibrium. A number that describes how far forward. Identify. What Is An Equilibrium Position.
From www.slideserve.com
PPT The Position of Equilibrium PowerPoint Presentation, free What Is An Equilibrium Position The position of equilibrium moves to the left. The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium. This is essentially what happens if one of the products is removed as soon as it is. The position of equilibrium is a property of the particular reversible reaction and does not depend upon. What Is An Equilibrium Position.
From www.slideserve.com
PPT Equilibrium Chemistry PowerPoint Presentation, free download ID What Is An Equilibrium Position The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium. A reversible reaction is one which can be made to go. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. A particular set of concentrations of reactant and product species. Identify the physical. What Is An Equilibrium Position.
From gamesmartz.com
Equilibrium Position Definition & Image GameSmartz What Is An Equilibrium Position This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. A reversible reaction is one which can be made to go. The position of equilibrium moves to the left. A particular. What Is An Equilibrium Position.
From www.slideshare.net
Physiology of equilibrium & balance What Is An Equilibrium Position The position of equilibrium moves to the left. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. A number that describes how far forward. Identify the physical conditions of static. What Is An Equilibrium Position.
From www.doubtnut.com
Figure shown a spring block system hanging in equilibrium. The block o What Is An Equilibrium Position A particular set of concentrations of reactant and product species. A reversible reaction is one which can be made to go. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. The position of equilibrium moves to the left. The position of equilibrium is a property of the particular reversible reaction and. What Is An Equilibrium Position.
From www.youtube.com
Position of Equilibrium YouTube What Is An Equilibrium Position The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium. This is essentially what happens if one of the products is removed as soon as it is. The position of equilibrium moves to the left. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium. What Is An Equilibrium Position.
From www.chemicals.co.uk
Chemistry A Level Revision Equilibrium What Is An Equilibrium Position The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium. A particular set of concentrations of reactant and product species. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. A number that describes how far forward. The position of equilibrium. What Is An Equilibrium Position.
From www.nagwa.com
Question Video Identifying What Is Meant by the Equilibrium Position What Is An Equilibrium Position Explain how the conditions for equilibrium. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. The position of equilibrium moves to the left. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. A reversible reaction is one which can. What Is An Equilibrium Position.
From courses.lumenlearning.com
Conditions for Equilibrium Boundless Physics What Is An Equilibrium Position Identify the physical conditions of static equilibrium. A reversible reaction is one which can be made to go. This is essentially what happens if one of the products is removed as soon as it is. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. Explain how the conditions. What Is An Equilibrium Position.
From www.youtube.com
Class 9Physics States of Equilibrium YouTube What Is An Equilibrium Position Identify the physical conditions of static equilibrium. A reversible reaction is one which can be made to go. The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium. This is essentially what happens if one of the products is removed as soon as it is. A number that describes how far forward.. What Is An Equilibrium Position.
From www.slideserve.com
PPT Topic 07 Equilibrium 7.1 Dynamic Equilibrium PowerPoint What Is An Equilibrium Position Explain how the conditions for equilibrium. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. A reversible reaction is one which can be made to go. This is essentially what happens if one of the products is removed as soon as it is. Identify the physical conditions of. What Is An Equilibrium Position.
From www.slideshare.net
Equilibrium What Is An Equilibrium Position Explain how the conditions for equilibrium. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. This is essentially what happens if one of the products is removed as soon as it is. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium. What Is An Equilibrium Position.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is An Equilibrium Position A particular set of concentrations of reactant and product species. The position of equilibrium moves to the left. The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. Identify the physical. What Is An Equilibrium Position.
From www.investopedia.com
Equilibrium Quantity Definition What Is An Equilibrium Position A reversible reaction is one which can be made to go. A number that describes how far forward. Explain how the conditions for equilibrium. The position of equilibrium moves to the left. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. Identify the physical conditions of static equilibrium.. What Is An Equilibrium Position.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID5729485 What Is An Equilibrium Position A number that describes how far forward. Explain how the conditions for equilibrium. The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium. The position of equilibrium moves to the left. Identify the physical conditions of static equilibrium. A particular set of concentrations of reactant and product species. This leads to the. What Is An Equilibrium Position.
From www.youtube.com
Equilibrium of Forces A level Physics YouTube What Is An Equilibrium Position A reversible reaction is one which can be made to go. Identify the physical conditions of static equilibrium. A particular set of concentrations of reactant and product species. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. The position of equilibrium is a property of the particular reversible reaction and does. What Is An Equilibrium Position.
From www.slideserve.com
PPT Parts of a Wave PowerPoint Presentation, free download ID6857698 What Is An Equilibrium Position The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. This is essentially what happens if one of the products is removed as soon as it is. A reversible reaction is one which can be made to go. The position of equilibrium (also called equilibrium position) describes the relative. What Is An Equilibrium Position.
From www.slideserve.com
PPT Ch. 13 Chemical Equilibrium PowerPoint Presentation, free What Is An Equilibrium Position Identify the physical conditions of static equilibrium. A reversible reaction is one which can be made to go. A number that describes how far forward. This is essentially what happens if one of the products is removed as soon as it is. The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium.. What Is An Equilibrium Position.
From www.youtube.com
Simple Harmonic Motion IB Physics YouTube What Is An Equilibrium Position The position of equilibrium moves to the left. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. This is essentially what happens if one of the products is removed as soon as it is. A number that describes how far forward. A particular set of concentrations of reactant. What Is An Equilibrium Position.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is An Equilibrium Position This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at equilibrium. Identify the physical conditions of static equilibrium. A reversible reaction is one which can be made to go. A particular set of concentrations of. What Is An Equilibrium Position.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is An Equilibrium Position This is essentially what happens if one of the products is removed as soon as it is. Explain how the conditions for equilibrium. The position of equilibrium moves to the left. A reversible reaction is one which can be made to go. A particular set of concentrations of reactant and product species. This leads to the idea of a dynamic. What Is An Equilibrium Position.
From www.slideserve.com
PPT What are acids and alkalis? PowerPoint Presentation, free What Is An Equilibrium Position This is essentially what happens if one of the products is removed as soon as it is. Explain how the conditions for equilibrium. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. This leads to the idea of a dynamic equilibrium, and what the common term position of. What Is An Equilibrium Position.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is An Equilibrium Position Identify the physical conditions of static equilibrium. The position of equilibrium moves to the left. A reversible reaction is one which can be made to go. A number that describes how far forward. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. A particular set of concentrations of reactant and product. What Is An Equilibrium Position.
From www.len.com.ng
Static and Dynamic Equilibrium explained with their differences What Is An Equilibrium Position Explain how the conditions for equilibrium. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. The position of equilibrium moves to the left. Identify the physical conditions of static equilibrium.. What Is An Equilibrium Position.
From www.javierparra.net
Contents, Economics General equilibrium theory What Is An Equilibrium Position This is essentially what happens if one of the products is removed as soon as it is. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. The position of equilibrium. What Is An Equilibrium Position.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID2633301 What Is An Equilibrium Position This is essentially what happens if one of the products is removed as soon as it is. This leads to the idea of a dynamic equilibrium, and what the common term position of equilibrium means. The position of equilibrium moves to the left. The position of equilibrium (also called equilibrium position) describes the relative concentrations of reactants and products at. What Is An Equilibrium Position.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is An Equilibrium Position The position of equilibrium is a property of the particular reversible reaction and does not depend upon how equilibrium was achieved. This is essentially what happens if one of the products is removed as soon as it is. A particular set of concentrations of reactant and product species. Explain how the conditions for equilibrium. This leads to the idea of. What Is An Equilibrium Position.