Zinc Bromide Oxidation State . Zinc bromide = zinc + bromine. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. The following chart describes the most common oxidation states of the period 3 elements. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. A net ionic charge can be specified at the end of the. Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. Enter the formula of a chemical compound to find the oxidation number of each element.
from www.youtube.com
The following chart describes the most common oxidation states of the period 3 elements. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. A net ionic charge can be specified at the end of the. Zinc bromide = zinc + bromine. In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. Enter the formula of a chemical compound to find the oxidation number of each element. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes.
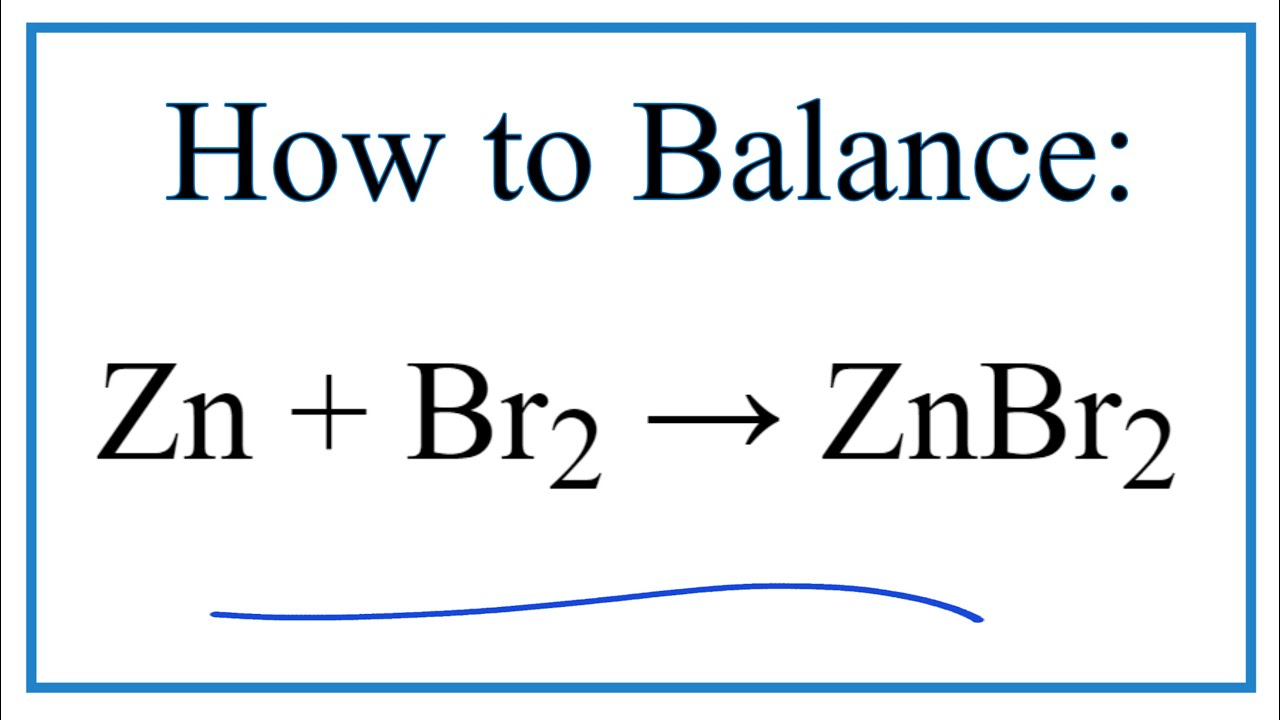
How to Balance Zn + Br2 = ZnBr2 (Zinc + Bromine gas) YouTube
Zinc Bromide Oxidation State In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. Zinc bromide = zinc + bromine. Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be specified at the end of the. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. The following chart describes the most common oxidation states of the period 3 elements. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to.
From chem.libretexts.org
3.4 Oxidation States Chemistry LibreTexts Zinc Bromide Oxidation State Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. Zinc bromide = zinc + bromine. 119 rows the oxidation state tells how many valence. Zinc Bromide Oxidation State.
From www.youtube.com
Equation for ZnCl2 + H2O (Zinc chloride + Water) YouTube Zinc Bromide Oxidation State In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. A net ionic charge can be specified at the end of the. Znbr2 = zn. Zinc Bromide Oxidation State.
From www.slideserve.com
PPT OXIDATION AND REDUCTION PowerPoint Presentation, free download Zinc Bromide Oxidation State Zinc bromide = zinc + bromine. Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be specified at the end of the. Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. 119 rows the oxidation state tells how many. Zinc Bromide Oxidation State.
From www.slideserve.com
PPT What is electrolysis? PowerPoint Presentation, free download ID Zinc Bromide Oxidation State The following chart describes the most common oxidation states of the period 3 elements. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. Zinc bromide = zinc + bromine. Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be. Zinc Bromide Oxidation State.
From chem.libretexts.org
10.6.2. Strong Oxidizing Agents Chemistry LibreTexts Zinc Bromide Oxidation State Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. The following chart describes the most common oxidation states of the period 3 elements. Enter the formula of a chemical compound to. Zinc Bromide Oxidation State.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc Bromide Oxidation State Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. Enter the formula of a chemical compound to find the oxidation number of each element. The following chart describes the most common oxidation states of the period 3 elements. 119 rows the oxidation state tells how many valence electrons an atom. Zinc Bromide Oxidation State.
From www.indiamart.com
Zinc Bromide LR & AR Grade at best price in Palghar by Xena Organics Zinc Bromide Oxidation State A net ionic charge can be specified at the end of the. Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. Enter the formula of a chemical compound to find the oxidation number of each element. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative. Zinc Bromide Oxidation State.
From www.pw.live
Zinc Bromide Formula, Structure And Properties Zinc Bromide Oxidation State 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. Enter the formula of a chemical compound to find the oxidation number of each element. The following chart describes the most common oxidation states of the period 3 elements. A net ionic charge can be specified at the end of. Zinc Bromide Oxidation State.
From www.fishersci.com
Zinc bromide hydrate, 99.9 (metals basis), Thermo Scientific Chemicals Zinc Bromide Oxidation State In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc. Zinc Bromide Oxidation State.
From www.youtube.com
How to find the Oxidation Numbers for Zn(NO3)2 (Zinc nitrate) YouTube Zinc Bromide Oxidation State Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. Zinc bromide = zinc + bromine. The following chart describes the most common oxidation states of the period 3 elements. Enter. Zinc Bromide Oxidation State.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) Redox Zinc Bromide Oxidation State Scandium is one of the two elements in the first transition metal period which has only one oxidation state. A net ionic charge can be specified at the end of the. In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. Enter the formula. Zinc Bromide Oxidation State.
From www.youtube.com
How to Draw the Lewis Dot Structure for ZnBr2 (Zinc bromide) YouTube Zinc Bromide Oxidation State Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. A net ionic charge can be specified at the end of the. Scandium is one of the two elements in the. Zinc Bromide Oxidation State.
From www.numerade.com
SOLVED For the following reactions, write out the reaction and use Zinc Bromide Oxidation State Scandium is one of the two elements in the first transition metal period which has only one oxidation state. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. The following chart describes the most common oxidation states of the period 3 elements. Znbr2 = zn + br is a. Zinc Bromide Oxidation State.
From www.grainger.com
7699458, F.W. 225.21, Zinc Bromide, Anhydrous, Reagent 39H151Z1048 Zinc Bromide Oxidation State The following chart describes the most common oxidation states of the period 3 elements. Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. A net ionic charge can be specified at the end of the. Zinc bromide = zinc + bromine. Scandium is one of the two elements in the. Zinc Bromide Oxidation State.
From www.mdpi.com
Energies Free FullText Operational Parameter Analysis and Zinc Bromide Oxidation State A net ionic charge can be specified at the end of the. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. Zinc bromide = zinc + bromine. The following chart. Zinc Bromide Oxidation State.
From byjus.com
Briefly explain the process of electrolysis of molten Lead Bromide Zinc Bromide Oxidation State The following chart describes the most common oxidation states of the period 3 elements. Zinc bromide = zinc + bromine. A net ionic charge can be specified at the end of the. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. In chemistry, the oxidation state, or oxidation number,. Zinc Bromide Oxidation State.
From www.slideserve.com
PPT Corrosion of Metals PowerPoint Presentation, free download ID Zinc Bromide Oxidation State In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. The following chart describes the most common oxidation states of the period 3 elements.. Zinc Bromide Oxidation State.
From www.fishersci.com
Zinc bromide, 98, Acros OrganicsChemicalsOther Compounds Zinc Bromide Oxidation State A net ionic charge can be specified at the end of the. Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. Enter the formula of a chemical compound to find. Zinc Bromide Oxidation State.
From www.youtube.com
How to find the Oxidation Number for Zn (Zinc) YouTube Zinc Bromide Oxidation State Scandium is one of the two elements in the first transition metal period which has only one oxidation state. A net ionic charge can be specified at the end of the. In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. The following chart. Zinc Bromide Oxidation State.
From www.indiamart.com
Zinc Bromide solution 70 at Rs 190/kgs ZnBr2 in Ankleshwar ID Zinc Bromide Oxidation State Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. Zinc bromide = zinc + bromine. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. A net ionic charge can be specified at the end of the. The following chart. Zinc Bromide Oxidation State.
From www.pngwing.com
Zincbromine battery Zinc bromide Flow battery, others, chemistry Zinc Bromide Oxidation State Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. The following. Zinc Bromide Oxidation State.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Zinc Bromide Oxidation State Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. Enter the formula of a chemical compound to find the oxidation number of each element. 119 rows the oxidation state tells how. Zinc Bromide Oxidation State.
From www.numerade.com
SOLVED The compound zinc bromide, ZnBr2 is soluble in water. Write the Zinc Bromide Oxidation State 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. Enter the formula of a chemical compound to find the oxidation number of each. Zinc Bromide Oxidation State.
From www.numerade.com
SOLVED Aqueous solutions of zinc bromide and sodium phosphate combine Zinc Bromide Oxidation State Enter the formula of a chemical compound to find the oxidation number of each element. Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. Zinc bromide = zinc + bromine. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. A. Zinc Bromide Oxidation State.
From www.britannica.com
Zinc Properties, Uses, & Facts Britannica Zinc Bromide Oxidation State A net ionic charge can be specified at the end of the. Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. In chemistry, the oxidation state, or oxidation number, is the. Zinc Bromide Oxidation State.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc Bromide Oxidation State Scandium is one of the two elements in the first transition metal period which has only one oxidation state. Zinc bromide = zinc + bromine. The following chart describes the most common oxidation states of the period 3 elements. In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to. Zinc Bromide Oxidation State.
From dir.indiamart.com
Zinc Bromide ZnBr2 Latest Price, Manufacturers & Suppliers Zinc Bromide Oxidation State In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. A net ionic charge can be specified at the end of the. Zinc bromide = zinc + bromine. Scandium is one of the two elements in the first transition metal period which has only. Zinc Bromide Oxidation State.
From www.fishersci.fi
Zinc bromide, 99.999, (trace metal basis), extra pure, Thermo Zinc Bromide Oxidation State The following chart describes the most common oxidation states of the period 3 elements. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. Enter. Zinc Bromide Oxidation State.
From www.youtube.com
How to Balance Zn + Br2 = ZnBr2 (Zinc + Bromine gas) YouTube Zinc Bromide Oxidation State In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. The following chart describes the most common oxidation states of the period 3 elements. Zinc bromide = zinc + bromine. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative. Zinc Bromide Oxidation State.
From www.glentham.com
Zinc bromide, anhydrous, 99 (CAS 7699458) Glentham Life Sciences Zinc Bromide Oxidation State The following chart describes the most common oxidation states of the period 3 elements. A net ionic charge can be specified at the end of the. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. Zinc bromide = zinc + bromine. In chemistry, the oxidation state, or oxidation number,. Zinc Bromide Oxidation State.
From www.toppr.com
Consider the change in oxidation state of Bromine corresponding to Zinc Bromide Oxidation State The following chart describes the most common oxidation states of the period 3 elements. In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to.. Zinc Bromide Oxidation State.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Zinc Bromide Oxidation State Scandium is one of the two elements in the first transition metal period which has only one oxidation state. Zinc bromide = zinc + bromine. Znbr2 = zn + br is a decomposition reaction where one mole of aqueous zinc bromide [znbr 2] decomposes. In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if. Zinc Bromide Oxidation State.
From www.nagwa.com
Question Video Describing How Oxidation Changes a Chemical Species in Zinc Bromide Oxidation State A net ionic charge can be specified at the end of the. Zinc bromide = zinc + bromine. The following chart describes the most common oxidation states of the period 3 elements. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. Enter the formula of a chemical compound to find. Zinc Bromide Oxidation State.
From www.adda247.com
What is the valency of zinc? Zinc Bromide Oxidation State In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. Scandium is one of the two elements in the first transition metal period which has only one oxidation state. Zinc bromide = zinc + bromine. The following chart describes the most common oxidation states. Zinc Bromide Oxidation State.
From www.expii.com
Color and Oxidation State — Overview & Examples Expii Zinc Bromide Oxidation State The following chart describes the most common oxidation states of the period 3 elements. A net ionic charge can be specified at the end of the. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to. Enter the formula of a chemical compound to find the oxidation number of each. Zinc Bromide Oxidation State.