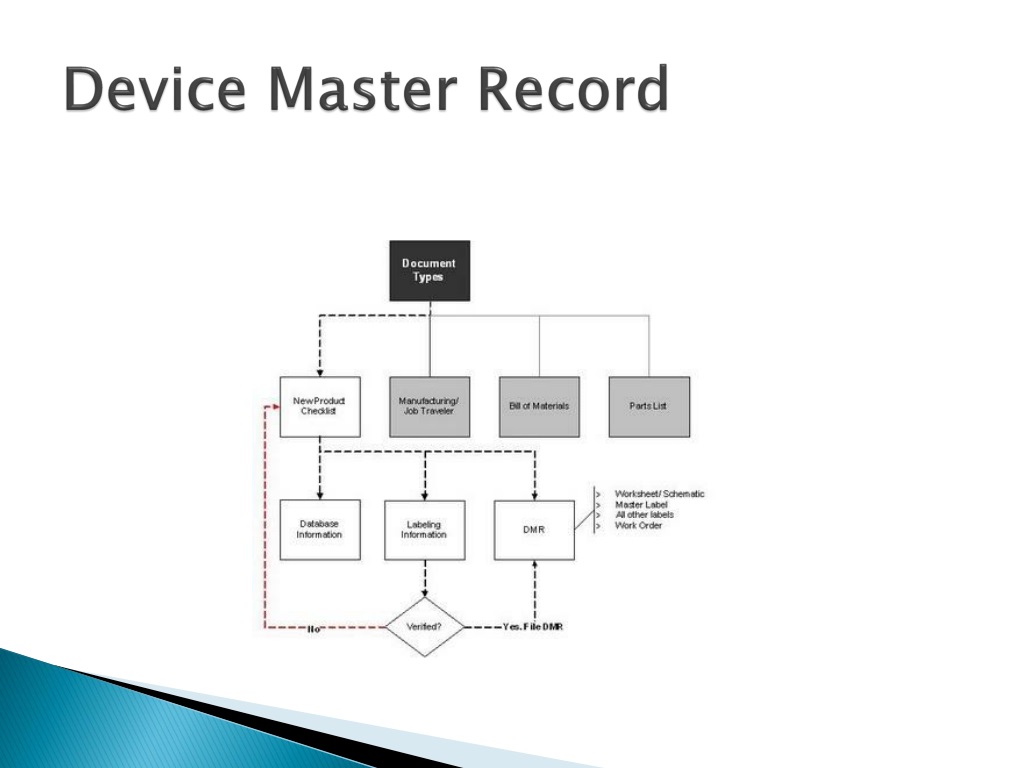
Device Master Record Example . When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in each. However, now includes a medical. The device master record is a regulatory requirement for all medical device companies. Fda requires the use of a device master record (dmr) for medical devices. A device master record is a collection of every document needed to manufacture, package, and possibly service a. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records.
from www.slideserve.com
A device master record is a collection of every document needed to manufacture, package, and possibly service a. Fda requires the use of a device master record (dmr) for medical devices. However, now includes a medical. Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in each. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. The device master record is a regulatory requirement for all medical device companies. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to.
PPT Design Documentation PowerPoint Presentation, free download ID
Device Master Record Example It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in each. When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. A device master record is a collection of every document needed to manufacture, package, and possibly service a. However, now includes a medical. The device master record is a regulatory requirement for all medical device companies. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. Fda requires the use of a device master record (dmr) for medical devices.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID Device Master Record Example Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in each. However, now includes a medical. Fda requires the use of a device master record (dmr) for medical devices. It is a repository of all essential information about your company’s medical devices and includes design requirements,. Device Master Record Example.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID Device Master Record Example A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. Fda requires the use of a device master record (dmr) for medical devices.. Device Master Record Example.
From www.bizmanualz.com
Device Master Record Procedure Template Word Device Master Record Example A device master record is a collection of every document needed to manufacture, package, and possibly service a. However, now includes a medical. The device master record is a regulatory requirement for all medical device companies. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and. Device Master Record Example.
From studylib.net
Device Master Record Device Master Record Example It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. Learn the subtle differences between design history file (dhf), device master record (dmr). Device Master Record Example.
From hardcoreqms.com
Device Master Records (DMR) for Medical Devices (2023) Device Master Record Example The device master record is a regulatory requirement for all medical device companies. Fda requires the use of a device master record (dmr) for medical devices. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. However, now includes a medical. When your device is in production your dmr should be the. Device Master Record Example.
From www.youtube.com
Preparing a Device Master Record (DMR) YouTube Device Master Record Example However, now includes a medical. Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in each. Fda requires the use of a device master record (dmr) for medical devices. It is a repository of all essential information about your company’s medical devices and includes design requirements,. Device Master Record Example.
From www.bizmanualz.com
Device Master Record Index Template Word Device Master Record Example Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in each. Fda requires the use of a device master record (dmr) for medical devices. The device master record is a regulatory requirement for all medical device companies. A device master record is a collection of every. Device Master Record Example.
From arrotek.com
Device Master Record What Is It, and Why Is It Important? Arrotek Device Master Record Example The device master record is a regulatory requirement for all medical device companies. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in each. However, now includes a. Device Master Record Example.
From denner-shop-test-web02.denner.ch
Device Master Record Template Device Master Record Example A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. Fda requires the use of a device master record (dmr) for medical devices.. Device Master Record Example.
From www.scribd.com
Device Master Records.doc Specification (Technical Standard Device Master Record Example A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in each. It is a repository of all essential information about your company’s medical devices and includes design requirements,. Device Master Record Example.
From apilopas.weebly.com
Sample Device Master File apilopas Device Master Record Example The device master record is a regulatory requirement for all medical device companies. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. However, now includes a medical. A device master record is a collection of every document needed to manufacture,. Device Master Record Example.
From www.slideserve.com
PPT Product Documentation PowerPoint Presentation, free download ID Device Master Record Example It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. A device master record is a collection of every document needed to manufacture,. Device Master Record Example.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID Device Master Record Example Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in each. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. The device master record is a regulatory requirement for all medical device companies. When your device is. Device Master Record Example.
From www.presentationeze.com
Device Master Record DMR Information & Training.PresentationEZE Device Master Record Example The device master record is a regulatory requirement for all medical device companies. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. Fda requires the use of a device master record (dmr) for medical devices. It is a repository of all essential information about your company’s medical devices and includes design. Device Master Record Example.
From www.vrogue.co
Device Master Records Design History Files Gmp Docs vrogue.co Device Master Record Example When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. However, now includes a medical. A device master record (dmr). Device Master Record Example.
From denner-shop-test-web02.denner.ch
Device Master Record Template Device Master Record Example It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. However, now includes a medical. When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. Learn the subtle differences between. Device Master Record Example.
From alatpresstutupgelasplastikmurah160.blogspot.com
Medical Device Master File Template Device Master Record Example The device master record is a regulatory requirement for all medical device companies. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. When your device is in production your dmr should be the definitive record of all your manufacturing and. Device Master Record Example.
From www.technia.co.uk
What is a Device Master Record? TECHNIA (UK) Device Master Record Example When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. Fda requires the use of a device master record (dmr) for medical devices. Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in each. The. Device Master Record Example.
From www.bizmanualz.com
Device Master Record Contents Template Word Device Master Record Example A device master record is a collection of every document needed to manufacture, package, and possibly service a. Fda requires the use of a device master record (dmr) for medical devices. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. However, now includes a medical. Learn the subtle differences between design. Device Master Record Example.
From www.arenasolutions.com
Managing The Device Master Record (DMR) Arena Device Master Record Example However, now includes a medical. The device master record is a regulatory requirement for all medical device companies. Fda requires the use of a device master record (dmr) for medical devices. When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. A device master record is a collection of. Device Master Record Example.
From www.arenasolutions.com
Managing The Device Master Record (DMR) to Comply with 21 CFR Part 11 Device Master Record Example A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. However, now includes a medical. The device master record is a regulatory requirement for all medical device companies. A device master record is a collection of every document needed to manufacture, package, and possibly service a. It is a repository of all. Device Master Record Example.
From www.arenasolutions.com
Managing The Device Master Record (DMR) Arena Device Master Record Example The device master record is a regulatory requirement for all medical device companies. Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in each. However, now includes a medical. It is a repository of all essential information about your company’s medical devices and includes design requirements,. Device Master Record Example.
From www.bizmanualz.com
Device Master Record Procedure Template Word Device Master Record Example The device master record is a regulatory requirement for all medical device companies. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to.. Device Master Record Example.
From www.presentationeze.com
Device Master Record (DMR) What needs to be recorded into the DMR Device Master Record Example When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. However, now includes a medical. Fda requires the use of a device master record (dmr) for medical devices. The device master record is a regulatory requirement for all medical device companies. A device master record is a collection of. Device Master Record Example.
From www.technia.co.uk
What is a Device Master Record? TECHNIA (UK) Device Master Record Example When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. Fda requires the use of a device master record (dmr) for medical devices. A device master record is a collection of every document needed to manufacture, package, and possibly service a. Learn the subtle differences between design history file. Device Master Record Example.
From www.technia.co.jp
What is a Device Master Record? TECHNIA (Japan) Device Master Record Example The device master record is a regulatory requirement for all medical device companies. Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in each. Fda requires the use of a device master record (dmr) for medical devices. It is a repository of all essential information about. Device Master Record Example.
From old.sermitsiaq.ag
Device Master Record Template Device Master Record Example A device master record is a collection of every document needed to manufacture, package, and possibly service a. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. When your device is in production your dmr should be the definitive record. Device Master Record Example.
From elsmar.com
Index of /Cove_Premium/DMR Device Master Record Procedure Example/ Device Master Record Example Fda requires the use of a device master record (dmr) for medical devices. The device master record is a regulatory requirement for all medical device companies. A device master record is a collection of every document needed to manufacture, package, and possibly service a. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications. Device Master Record Example.
From templates.rjuuc.edu.np
Medical Device Design History File Template Device Master Record Example A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. Fda requires the use of a device master record (dmr) for medical devices. However, now includes a medical. The device master record is a regulatory requirement for all medical device companies. It is a repository of all essential information about your company’s. Device Master Record Example.
From www.youtube.com
Design History File (DHF), the Device Master Record (DMR) and the Device Master Record Example When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. However, now includes a medical. The device master record is a regulatory requirement for all medical device companies. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance. Device Master Record Example.
From templates.rjuuc.edu.np
Device History Record Template Device Master Record Example A device master record is a collection of every document needed to manufacture, package, and possibly service a. However, now includes a medical. The device master record is a regulatory requirement for all medical device companies. Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in. Device Master Record Example.
From www.technia.com
What is a Device Master Record? TECHNIA Device Master Record Example However, now includes a medical. When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. Learn the subtle differences between design history file (dhf), device master record (dmr) & device history. Device Master Record Example.
From www.qualitymeddev.com
Device Master Record Overview of FDA Requiements Device Master Record Example When your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. The device master record is a regulatory requirement for all medical device companies. Learn the subtle differences between design history file (dhf), device master record (dmr) & device history record (dhr) and which documents to include in each. A. Device Master Record Example.
From elsmar.com
Index of /Cove_Premium/DMR Device Master Record Procedure Example/ Device Master Record Example A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. A device master record is a collection of every document needed to manufacture,. Device Master Record Example.
From www.bizmanualz.com
Device Master Record Index Template Device Master Record Example A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to. It is a repository of all essential information about your company’s medical devices and includes design requirements, production requirements, quality assurance requirements, packaging and labeling specifications, and other records. However, now includes a medical. Fda requires the use of a device master. Device Master Record Example.