What Mean Atomic Weight . Atomic mass, the quantity of matter contained in an atom of an element. Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Since 1961 the standard unit. Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element.
from www.alamy.com
Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. Atomic mass, the quantity of matter contained in an atom of an element. The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Since 1961 the standard unit.
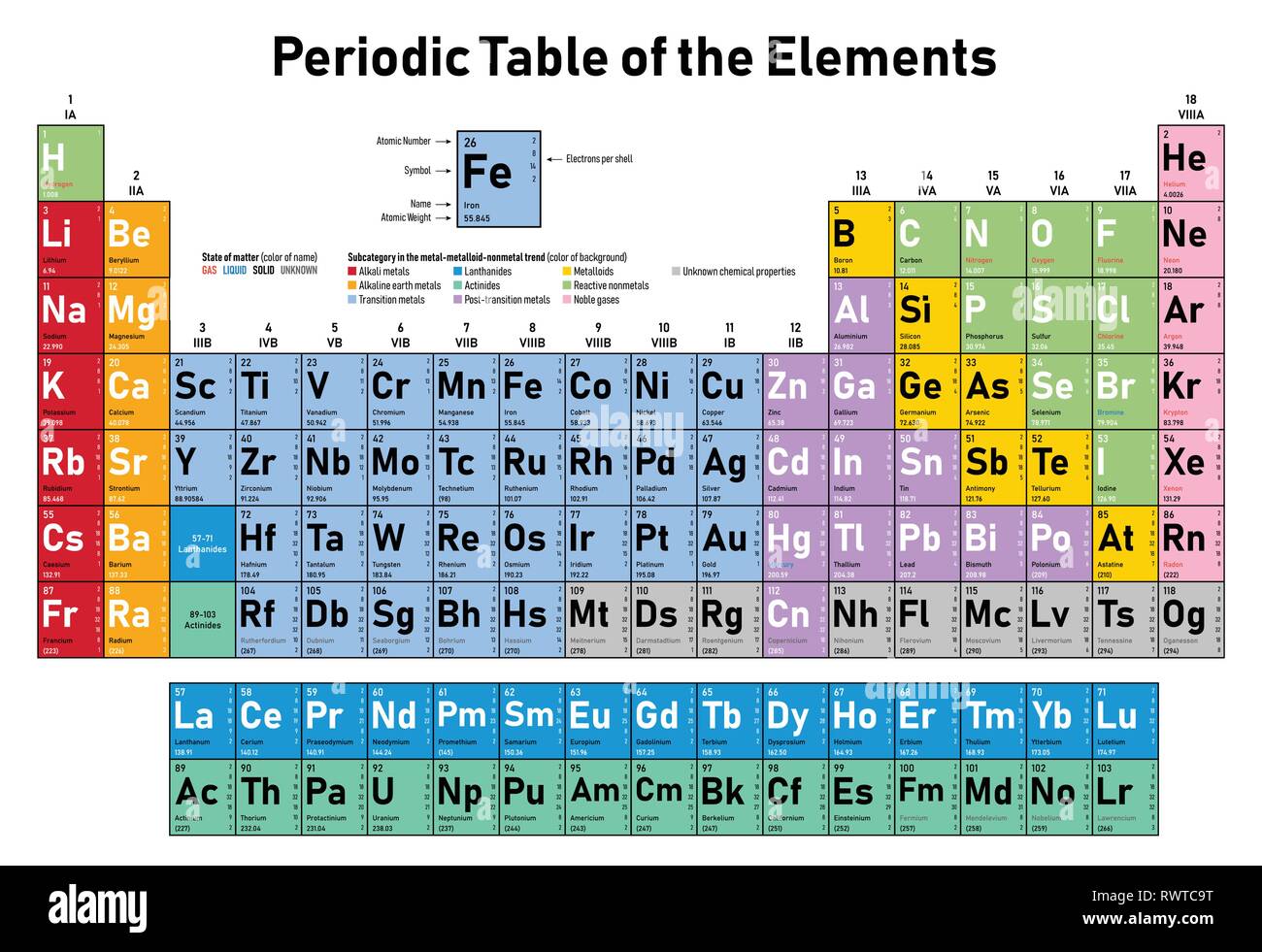
Colorful Periodic Table of the Elements shows atomic number, symbol
What Mean Atomic Weight The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. Since 1961 the standard unit. Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. Atomic mass, the quantity of matter contained in an atom of an element. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element.
From cen.acs.org
Revised Atomic Weights What Mean Atomic Weight The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an. What Mean Atomic Weight.
From www.wikihow.com
3 Clear and Easy Ways to Calculate Atomic Mass wikiHow What Mean Atomic Weight Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. Atomic mass is a property unique to each. What Mean Atomic Weight.
From www.mimprovement.com
Atomic number and atomic weight, Isoelectronic, Isobar, Isotones, Ions What Mean Atomic Weight Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms. What Mean Atomic Weight.
From www.youtube.com
How To Calculate The Average Atomic Mass YouTube What Mean Atomic Weight 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Atomic mass, the quantity of matter contained in an atom of an element. The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. Since 1961 the standard unit. Atomic mass, which is. What Mean Atomic Weight.
From www.wikihow.com
How to Calculate Molecular Weight 6 Steps (with Pictures) What Mean Atomic Weight Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. 121 rows atomic weight, ratio of the average mass. What Mean Atomic Weight.
From 139.59.164.119
Periodic Table with Atomic Mass What Mean Atomic Weight Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. Since 1961 the standard unit. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. The. What Mean Atomic Weight.
From www.slideserve.com
PPT What is atomic mass? PowerPoint Presentation, free download ID What Mean Atomic Weight Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Since 1961 the standard unit. The. What Mean Atomic Weight.
From www.youtube.com
Calculating average atomic mass of elements Tutorial YouTube What Mean Atomic Weight Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. The atomic weight can change because it depends. What Mean Atomic Weight.
From sciencenotes.org
What Is an Atomic Number? Definition and Examples What Mean Atomic Weight Atomic mass, the quantity of matter contained in an atom of an element. Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. Atomic weight. What Mean Atomic Weight.
From www.youtube.com
What does Atomic weight mean? YouTube What Mean Atomic Weight Atomic mass, the quantity of matter contained in an atom of an element. The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Atomic mass is a property unique to each element,. What Mean Atomic Weight.
From www.thoughtco.com
Difference Between Atomic Weight and Atomic Mass What Mean Atomic Weight Since 1961 the standard unit. Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. Atomic mass, the quantity of matter contained in an atom of an element. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. The. What Mean Atomic Weight.
From www.dreamstime.com
Background of the Periodic Table of the Chemical Elements with Their What Mean Atomic Weight Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Since 1961 the standard unit. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Atomic weight is a weighted average of the. What Mean Atomic Weight.
From www.pw.live
Difference Between Atomic Weight And Atomic Mass What Mean Atomic Weight The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Since 1961 the standard unit. Atomic mass is a property unique to each element, while atomic weight is a value that can. What Mean Atomic Weight.
From mungfali.com
Periodic Table Of Elements With Atomic Weight What Mean Atomic Weight 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. The atomic weight can change because it depends on our understanding of how. What Mean Atomic Weight.
From newtondesk.com
Explain Atomic Number And Mass Number of Element Chemistry What Mean Atomic Weight 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Atomic mass, the quantity of matter contained in an atom of an element. Since 1961 the standard unit. Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. Atomic. What Mean Atomic Weight.
From www.expii.com
How to Read the Periodic Table — Overview & Components Expii What Mean Atomic Weight Atomic mass, the quantity of matter contained in an atom of an element. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard.. What Mean Atomic Weight.
From www.youtube.com
Atomic weight Meaning YouTube What Mean Atomic Weight Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms. What Mean Atomic Weight.
From pediaa.com
Difference Between Atomic Number and Atomic Weight Definition What Mean Atomic Weight Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. Atomic mass, the quantity of matter contained in an atom of an element. The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. 121 rows atomic weight,. What Mean Atomic Weight.
From www.alamy.com
Colorful Periodic Table of the Elements shows atomic number, symbol What Mean Atomic Weight Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Atomic weight is a weighted average. What Mean Atomic Weight.
From www.slideserve.com
PPT Atomic Weights PowerPoint Presentation, free download ID3197304 What Mean Atomic Weight Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. The atomic weight can change because it depends on our understanding of how much of each isotope of an. What Mean Atomic Weight.
From www.slideserve.com
PPT Summary of the Atom PowerPoint Presentation, free download ID What Mean Atomic Weight Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. The atomic weight can change because. What Mean Atomic Weight.
From www.ssi.shimadzu.com
Molecular Weight Shimadzu Scientific Instruments What Mean Atomic Weight Atomic mass, the quantity of matter contained in an atom of an element. The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. Atomic mass,. What Mean Atomic Weight.
From www.slideserve.com
PPT Chem 1151 Ch. 2 PowerPoint Presentation, free download ID3579589 What Mean Atomic Weight Atomic mass, the quantity of matter contained in an atom of an element. Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. 121. What Mean Atomic Weight.
From www.thoughtco.com
Difference Between Atomic Mass and Mass Number What Mean Atomic Weight Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. The atomic weight can change because it depends. What Mean Atomic Weight.
From journeyz.co
What Are the Three Parts of an Atom? What Mean Atomic Weight Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. Since 1961 the standard unit. Atomic mass, the quantity of matter contained in an. What Mean Atomic Weight.
From www.slideserve.com
PPT Introduction to the Periodic Table PowerPoint Presentation, free What Mean Atomic Weight Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. Since 1961 the standard unit. The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. Atomic mass is a property unique to each element, while atomic weight. What Mean Atomic Weight.
From www.youtube.com
Atomic Weight and Average Atomic Mass Chemistry Tutorial YouTube What Mean Atomic Weight Atomic mass, the quantity of matter contained in an atom of an element. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. Atomic weight is a weighted average of the mass. What Mean Atomic Weight.
From www.youtube.com
Atomic Weight YouTube What Mean Atomic Weight Atomic mass, the quantity of matter contained in an atom of an element. Since 1961 the standard unit. Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element,. What Mean Atomic Weight.
From general.chemistrysteps.com
How To Calculate The Average Atomic Mass Chemistry Steps What Mean Atomic Weight Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Atomic mass, the quantity of matter contained in an atom of an element. Atomic mass is a property unique to each. What Mean Atomic Weight.
From www.britannica.com
periodic table Definition, Elements, Groups, Charges, Trends, & Facts What Mean Atomic Weight Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Since 1961 the standard unit. Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. The atomic weight. What Mean Atomic Weight.
From www.expii.com
Atomic Weight — Definition & Overview Expii What Mean Atomic Weight The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. Since 1961 the standard unit. Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. 121 rows atomic weight, ratio of the average mass of a chemical. What Mean Atomic Weight.
From www.researchgate.net
List of Elements with Range of Atomic Weights. Download Scientific What Mean Atomic Weight Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the. What Mean Atomic Weight.
From www.vecteezy.com
Periodic Table of the Elements shows atomic number, symbol, name What Mean Atomic Weight The atomic weight can change because it depends on our understanding of how much of each isotope of an element exists. Atomic mass is a property unique to each element, while atomic weight is a value that can vary depending on the abundance of isotopes. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to. What Mean Atomic Weight.
From elementtwentyseven.blogspot.com
LIST OF ATOMIC RADIUS AND ATOMIC WEIGHTS OF ELEMENTS BASIC INFORMATION What Mean Atomic Weight 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the. What Mean Atomic Weight.
From zakruti.com
Calculating atomic weight Chemistry What Mean Atomic Weight Atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. The atomic weight can change because it depends. What Mean Atomic Weight.