What Are Typical Metals . With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Remember that metals are on the left and bottom of the periodic table. They are one of three classes of elements (the other two classes are nonmetals and metalloids). Metals are elements that can conduct electricity. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. In the solid state, metals are composed of crystals made of closely packed atoms whose outer electrons are so loosely held that they are free to move throughout the crystal. Elements that are not metals. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Thus metals are electropositive elements with. List and explain the properties of metals.
from www.slideserve.com
Elements that are not metals. Remember that metals are on the left and bottom of the periodic table. List and explain the properties of metals. In the solid state, metals are composed of crystals made of closely packed atoms whose outer electrons are so loosely held that they are free to move throughout the crystal. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. They are one of three classes of elements (the other two classes are nonmetals and metalloids). Thus metals are electropositive elements with. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. Metals are elements that can conduct electricity.
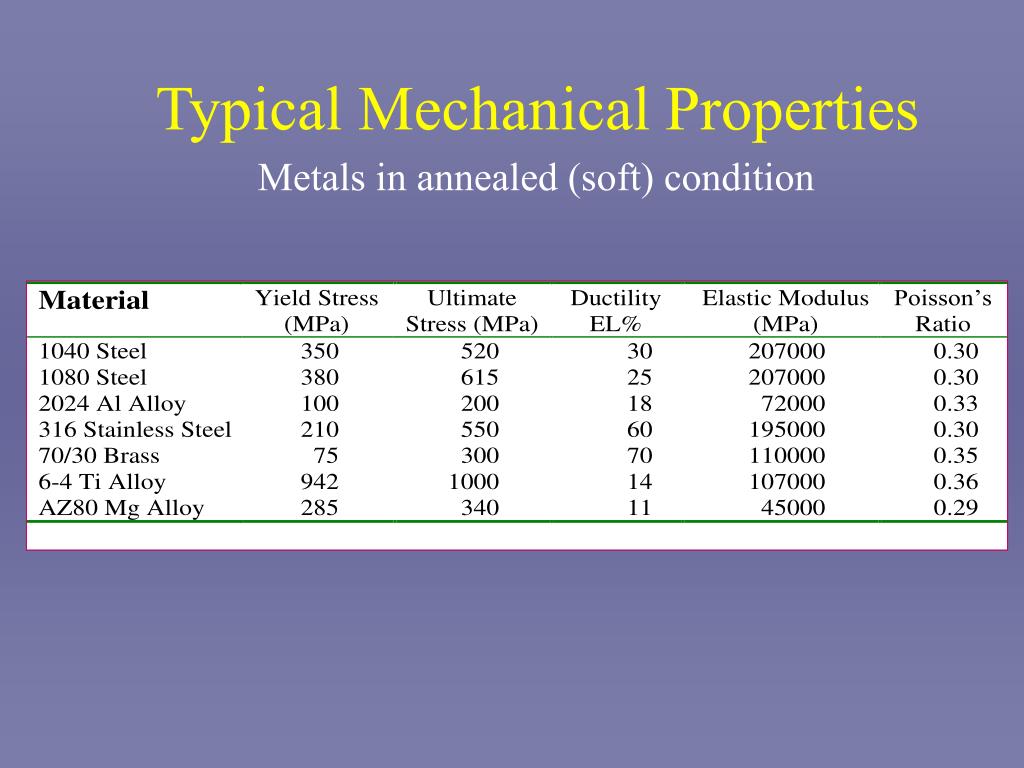
PPT Mechanical Properties of Metals PowerPoint Presentation, free
What Are Typical Metals Thus metals are electropositive elements with. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. They are one of three classes of elements (the other two classes are nonmetals and metalloids). Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. Metals are elements that can conduct electricity. Remember that metals are on the left and bottom of the periodic table. Elements that are not metals. Thus metals are electropositive elements with. List and explain the properties of metals. In the solid state, metals are composed of crystals made of closely packed atoms whose outer electrons are so loosely held that they are free to move throughout the crystal.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C2.2 ac Metals and nonmetals What Are Typical Metals Elements that are not metals. List and explain the properties of metals. Metals are elements that can conduct electricity. They are one of three classes of elements (the other two classes are nonmetals and metalloids). Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. The metals consist of the alkali. What Are Typical Metals.
From www.slideserve.com
PPT Mechanical Properties of Metals PowerPoint Presentation, free What Are Typical Metals Elements that are not metals. Remember that metals are on the left and bottom of the periodic table. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. They are one of three classes of elements (the other two classes are nonmetals and metalloids). Thus metals are electropositive elements with. List and explain the properties of. What Are Typical Metals.
From www.slideserve.com
PPT Metals and NonMetals PowerPoint Presentation ID1996394 What Are Typical Metals Remember that metals are on the left and bottom of the periodic table. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. In the solid state, metals are composed of crystals made of closely packed atoms whose outer electrons are so loosely held that they are free to move. What Are Typical Metals.
From studylib.net
Metals What Are Typical Metals Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. Thus metals are electropositive elements with. Metals are elements that can conduct electricity. Elements that are not metals. In the solid state, metals are composed of crystals made of closely packed atoms whose outer electrons are so loosely held that they. What Are Typical Metals.
From www.learnatnoon.com
What are the chemical properties of metals and nonmetals? Noon What Are Typical Metals They are one of three classes of elements (the other two classes are nonmetals and metalloids). Metals are elements that can conduct electricity. List and explain the properties of metals. In the solid state, metals are composed of crystals made of closely packed atoms whose outer electrons are so loosely held that they are free to move throughout the crystal.. What Are Typical Metals.
From madoverchemistry.wordpress.com
39.THE PERIODIC TABLE Metals. madoverchemistry What Are Typical Metals Elements that are not metals. Remember that metals are on the left and bottom of the periodic table. They are one of three classes of elements (the other two classes are nonmetals and metalloids). The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Metal, any of a class of substances characterized by high electrical and. What Are Typical Metals.
From blog.thepipingmart.com
How to Identify the Different Types of Metals An Overview What Are Typical Metals Elements that are not metals. List and explain the properties of metals. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. In the solid state, metals are composed of crystals made of closely packed atoms. What Are Typical Metals.
From www.pinterest.com
Pin by Storyteller Shivani on £¢learnenglish¥¶ Types of metal, Metal What Are Typical Metals Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. They are one of three classes of elements (the other two classes are nonmetals and metalloids). With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Thus metals are electropositive elements. What Are Typical Metals.
From slidetodoc.com
METALS Metals ferrous non ferrous alloys Ferrous metals What Are Typical Metals The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. They are one of three classes of elements (the other two classes are nonmetals and metalloids). Remember that metals are on the left and bottom of the periodic table. Metals are elements that can conduct electricity. List and explain the properties of metals. With the exception. What Are Typical Metals.
From www.thoughtco.com
The Difference Between Metals and Nonmetals What Are Typical Metals In the solid state, metals are composed of crystals made of closely packed atoms whose outer electrons are so loosely held that they are free to move throughout the crystal. Remember that metals are on the left and bottom of the periodic table. Elements that are not metals. Metals are elements that can conduct electricity. They are one of three. What Are Typical Metals.
From studymates91.blogspot.com
CBSE Class 8 Science Revision Notes Chapter 4 Materials Metals and Non What Are Typical Metals The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. List and explain the properties of metals. Thus metals are electropositive elements with. They are one of three classes of elements (the other two classes are nonmetals. What Are Typical Metals.
From www.stackumbrella.com
Top 10 Heaviest Metals on Earth Interesting Facts & Properties What Are Typical Metals The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Remember that metals are on the left and bottom of the periodic table. Metal, any of a class of substances characterized by high electrical and thermal. What Are Typical Metals.
From brainly.com
What are typical characteristics of metals What Are Typical Metals They are one of three classes of elements (the other two classes are nonmetals and metalloids). Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. Elements that are not metals. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. With the exception of hydrogen, all. What Are Typical Metals.
From pixels.com
Physical Properties Of Metals And Alloys Photograph by Inna Bigun What Are Typical Metals Remember that metals are on the left and bottom of the periodic table. Metals are elements that can conduct electricity. In the solid state, metals are composed of crystals made of closely packed atoms whose outer electrons are so loosely held that they are free to move throughout the crystal. The metals consist of the alkali metals, alkaline earths, transition. What Are Typical Metals.
From sciencenotes.org
Metals vs Nonmetals What Are Typical Metals They are one of three classes of elements (the other two classes are nonmetals and metalloids). Remember that metals are on the left and bottom of the periodic table. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. Metals are elements that can conduct electricity. The metals consist of the. What Are Typical Metals.
From www.slideserve.com
PPT Physical Properties of Metals PowerPoint Presentation ID5519685 What Are Typical Metals Elements that are not metals. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. They are. What Are Typical Metals.
From www.youtube.com
PHYSICAL PROPERTIES OF METALS METALS AND NON METALSPART 2 YouTube What Are Typical Metals Thus metals are electropositive elements with. Metals are elements that can conduct electricity. Elements that are not metals. List and explain the properties of metals. They are one of three classes of elements (the other two classes are nonmetals and metalloids). With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called. What Are Typical Metals.
From commons.wikimedia.org
FileTransition metals in the periodic table.png Wikimedia Commons What Are Typical Metals In the solid state, metals are composed of crystals made of closely packed atoms whose outer electrons are so loosely held that they are free to move throughout the crystal. Thus metals are electropositive elements with. Metals are elements that can conduct electricity. Remember that metals are on the left and bottom of the periodic table. The metals consist of. What Are Typical Metals.
From brainly.in
State any five physical properties on the basis of which metals can be What Are Typical Metals Metals are elements that can conduct electricity. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. They are one of three classes of elements (the other two classes are nonmetals and metalloids). List and explain the properties of metals. Thus metals are electropositive elements with. Remember that metals are on the left and bottom of. What Are Typical Metals.
From www.teachoo.com
Metals, Non Metals and Metalloids Meaning & Difference Teachoo What Are Typical Metals With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Elements that are not metals. They are one of three classes of elements (the other two classes are nonmetals and metalloids). Thus metals are electropositive elements with. Metals are elements that can conduct electricity. List and explain the properties of. What Are Typical Metals.
From www.slideserve.com
PPT Metals PowerPoint Presentation, free download ID3547992 What Are Typical Metals They are one of three classes of elements (the other two classes are nonmetals and metalloids). Thus metals are electropositive elements with. Metals are elements that can conduct electricity. Elements that are not metals. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. In the solid state, metals are composed of crystals made of closely. What Are Typical Metals.
From www.thoughtco.com
Metals Quiz Metal Properties and Chemistry What Are Typical Metals Metals are elements that can conduct electricity. In the solid state, metals are composed of crystals made of closely packed atoms whose outer electrons are so loosely held that they are free to move throughout the crystal. Remember that metals are on the left and bottom of the periodic table. The metals consist of the alkali metals, alkaline earths, transition. What Are Typical Metals.
From www.thoughtco.com
Properties of the Basic Metals Element Group What Are Typical Metals Elements that are not metals. List and explain the properties of metals. Thus metals are electropositive elements with. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. In the solid state, metals are composed of crystals. What Are Typical Metals.
From www.meadmetals.com
What’s the Difference Between Metals, Nonmetals, and Metalloids? What Are Typical Metals List and explain the properties of metals. Thus metals are electropositive elements with. Remember that metals are on the left and bottom of the periodic table. Metals are elements that can conduct electricity. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Metal, any of a class of substances characterized by high electrical and thermal. What Are Typical Metals.
From www.slideserve.com
PPT Metals and their Properties PowerPoint Presentation, free What Are Typical Metals With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Remember that metals are on the left and bottom of the periodic table. They are one of three classes of elements (the other two classes are nonmetals and metalloids). Thus metals are electropositive elements with. In the solid state, metals. What Are Typical Metals.
From co.pinterest.com
Pin on What Are Typical Metals Remember that metals are on the left and bottom of the periodic table. List and explain the properties of metals. They are one of three classes of elements (the other two classes are nonmetals and metalloids). Thus metals are electropositive elements with. In the solid state, metals are composed of crystals made of closely packed atoms whose outer electrons are. What Are Typical Metals.
From www.slideserve.com
PPT Chapter 6 / Unit 2 Section A Periodic Table of elements What Are Typical Metals With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Thus metals are electropositive elements with. List. What Are Typical Metals.
From engineeringlearn.com
16 Types of Metals and Their Uses [with Pictures] Engineering Learn What Are Typical Metals Metals are elements that can conduct electricity. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. Thus metals are electropositive elements with. List and explain the properties of metals. Elements. What Are Typical Metals.
From www.nuclear-power.com
Steels Properties of Steels What Are Typical Metals With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Elements that are not metals. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. They are one of three classes of elements (the other two classes are nonmetals and metalloids).. What Are Typical Metals.
From www.teachoo.com
Different Uses of Metals Explained with examples Teachoo What Are Typical Metals Thus metals are electropositive elements with. Elements that are not metals. With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. List and explain the properties of metals. They are one. What Are Typical Metals.
From www.vedantu.com
Alkali Metals Chemical Elements, Properties Alkali Metals Periodic What Are Typical Metals The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Thus metals are electropositive elements with. Remember that metals are on the left and bottom of the periodic table. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. In the solid state, metals are composed of. What Are Typical Metals.
From examples.yourdictionary.com
Basic Types of Metals on the Periodic Table What Are Typical Metals The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Metals are elements that can conduct electricity. List and explain the properties of metals. Remember that metals are on the left and bottom of the periodic table. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,.. What Are Typical Metals.
From www.mechical.com
Ferrous Metal Definition, Types, Uses, Properties What Are Typical Metals In the solid state, metals are composed of crystals made of closely packed atoms whose outer electrons are so loosely held that they are free to move throughout the crystal. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. List and explain the properties of metals. Metals are elements that can conduct electricity. Thus metals. What Are Typical Metals.
From ar.inspiredpencil.com
Transition Metals Group What Are Typical Metals With the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Elements that are not metals. Metal, any of a class of substances characterized by high electrical and thermal conductivity as well as by malleability,. List and. What Are Typical Metals.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.54C Explain Typical Physical Properties of What Are Typical Metals List and explain the properties of metals. They are one of three classes of elements (the other two classes are nonmetals and metalloids). The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides. Metals are elements that can conduct electricity. Thus metals are electropositive elements with. Metal, any of a class of substances characterized by high. What Are Typical Metals.