Chlorine Atom Has What Oxidation Number . The oxidation number for chlorine can vary depending on the compound it is. The oxidation number of an atom is defined as the charge that an atom. Allotropes some elements exist in several different structural forms, called allotropes. what is the oxidation number? Electron configuration [na]3s 2 3p 5: oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. what is the oxidation number for chlorine?
from www.scribd.com
Allotropes some elements exist in several different structural forms, called allotropes. the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. The oxidation number of an atom is defined as the charge that an atom. Electron configuration [na]3s 2 3p 5: The oxidation number for chlorine can vary depending on the compound it is. what is the oxidation number for chlorine? what is the oxidation number? oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or.
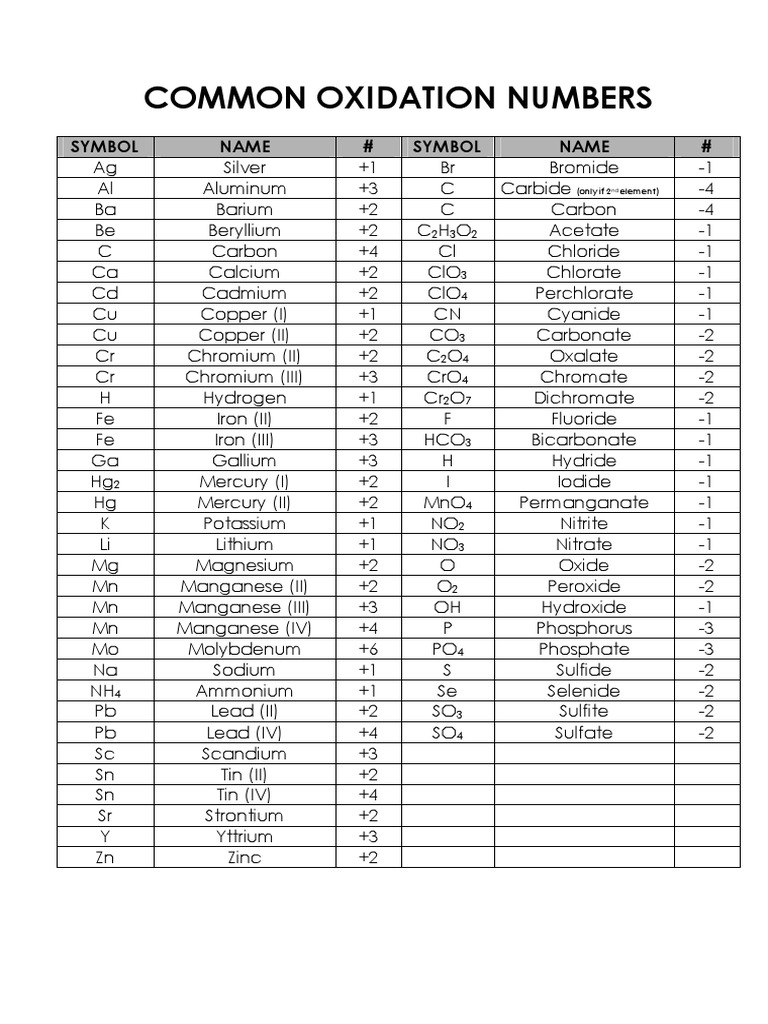
Common Oxidation Numbers Chart
Chlorine Atom Has What Oxidation Number The oxidation number of an atom is defined as the charge that an atom. The oxidation number of an atom is defined as the charge that an atom. the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. what is the oxidation number for chlorine? oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. Electron configuration [na]3s 2 3p 5: The oxidation number for chlorine can vary depending on the compound it is. Allotropes some elements exist in several different structural forms, called allotropes. what is the oxidation number?
From www.quora.com
Does Chlorine and Bromine exhibit +4 and +6 oxidation state in oxides Chlorine Atom Has What Oxidation Number oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. Electron configuration [na]3s 2 3p 5: Allotropes some elements exist in several different structural forms, called allotropes. what is the oxidation number? the oxidation state of an atom is equal to the total number of electrons which. Chlorine Atom Has What Oxidation Number.
From www.shalom-education.com
Covalent Bonding GCSE Chemistry Revision Chlorine Atom Has What Oxidation Number Allotropes some elements exist in several different structural forms, called allotropes. Electron configuration [na]3s 2 3p 5: what is the oxidation number? The oxidation number for chlorine can vary depending on the compound it is. the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a.. Chlorine Atom Has What Oxidation Number.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor Chlorine Atom Has What Oxidation Number The oxidation number of an atom is defined as the charge that an atom. oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. what is the oxidation number? Allotropes some elements exist in several different structural forms, called allotropes. what is the oxidation number for chlorine?. Chlorine Atom Has What Oxidation Number.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Atom Has What Oxidation Number The oxidation number of an atom is defined as the charge that an atom. the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. what is the oxidation number for chlorine? The oxidation number for chlorine can vary depending on the compound it is. Electron. Chlorine Atom Has What Oxidation Number.
From askfilo.com
Oxidation number of chlorine atoms in CaOCl2 are Filo Chlorine Atom Has What Oxidation Number Electron configuration [na]3s 2 3p 5: Allotropes some elements exist in several different structural forms, called allotropes. the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. what is the oxidation number for chlorine? The oxidation number for chlorine can vary depending on the compound. Chlorine Atom Has What Oxidation Number.
From askfilo.com
Oxidation states of chlorine in NOCl and OCl2 are Filo Chlorine Atom Has What Oxidation Number Electron configuration [na]3s 2 3p 5: the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. Allotropes some elements exist in several different structural forms, called allotropes. what is the oxidation number for chlorine? oxidation state shows the total number of electrons which have. Chlorine Atom Has What Oxidation Number.
From www.onlinechemistrytutor.net
chlorine001 Online Chemistry Tutor Chlorine Atom Has What Oxidation Number Electron configuration [na]3s 2 3p 5: The oxidation number of an atom is defined as the charge that an atom. Allotropes some elements exist in several different structural forms, called allotropes. The oxidation number for chlorine can vary depending on the compound it is. oxidation state shows the total number of electrons which have been removed from an element. Chlorine Atom Has What Oxidation Number.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor Chlorine Atom Has What Oxidation Number Allotropes some elements exist in several different structural forms, called allotropes. the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. Electron configuration [na]3s 2. Chlorine Atom Has What Oxidation Number.
From www.expii.com
Ions — Definition & Overview Expii Chlorine Atom Has What Oxidation Number what is the oxidation number for chlorine? the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. The oxidation number for chlorine can vary depending on the compound it is. Electron configuration [na]3s 2 3p 5: oxidation state shows the total number of electrons. Chlorine Atom Has What Oxidation Number.
From www.askiitians.com
Chlorine Study Material for IIT JEE askIITians Chlorine Atom Has What Oxidation Number oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. The oxidation number of an atom is defined as the charge that an atom. what is the oxidation number? Electron configuration [na]3s 2 3p 5: what is the oxidation number for chlorine? the oxidation state of. Chlorine Atom Has What Oxidation Number.
From www.youtube.com
How to find Oxidation Numbers for Chlorine (Cl and Cl2) YouTube Chlorine Atom Has What Oxidation Number Allotropes some elements exist in several different structural forms, called allotropes. Electron configuration [na]3s 2 3p 5: The oxidation number for chlorine can vary depending on the compound it is. oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. the oxidation state of an atom is equal. Chlorine Atom Has What Oxidation Number.
From www.bigstockphoto.com
Oxidation Numbers Image & Photo (Free Trial) Bigstock Chlorine Atom Has What Oxidation Number The oxidation number of an atom is defined as the charge that an atom. what is the oxidation number for chlorine? Allotropes some elements exist in several different structural forms, called allotropes. The oxidation number for chlorine can vary depending on the compound it is. Electron configuration [na]3s 2 3p 5: oxidation state shows the total number of. Chlorine Atom Has What Oxidation Number.
From www.chegg.com
Solved B. Determine the oxidation number of Cl a) HClO4 b) Chlorine Atom Has What Oxidation Number The oxidation number of an atom is defined as the charge that an atom. The oxidation number for chlorine can vary depending on the compound it is. what is the oxidation number? Electron configuration [na]3s 2 3p 5: the oxidation state of an atom is equal to the total number of electrons which have been removed from an. Chlorine Atom Has What Oxidation Number.
From cecruqqd.blob.core.windows.net
Chlorine Half Equation at Angela Villa blog Chlorine Atom Has What Oxidation Number The oxidation number for chlorine can vary depending on the compound it is. Electron configuration [na]3s 2 3p 5: the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. oxidation state shows the total number of electrons which have been removed from an element (a. Chlorine Atom Has What Oxidation Number.
From www.numerade.com
SOLVEDChlorine can have several different oxidation numbers ranging in Chlorine Atom Has What Oxidation Number what is the oxidation number? Electron configuration [na]3s 2 3p 5: The oxidation number of an atom is defined as the charge that an atom. what is the oxidation number for chlorine? The oxidation number for chlorine can vary depending on the compound it is. Allotropes some elements exist in several different structural forms, called allotropes. the. Chlorine Atom Has What Oxidation Number.
From momzik.weebly.com
Periodic table chemistry with oxidation numbers momzik Chlorine Atom Has What Oxidation Number The oxidation number for chlorine can vary depending on the compound it is. Allotropes some elements exist in several different structural forms, called allotropes. oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. The oxidation number of an atom is defined as the charge that an atom. . Chlorine Atom Has What Oxidation Number.
From www.sliderbase.com
Electrochemistry Presentation Chemistry Chlorine Atom Has What Oxidation Number The oxidation number of an atom is defined as the charge that an atom. oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. The oxidation number for chlorine can vary depending on the compound it is. what is the oxidation number for chlorine? the oxidation state. Chlorine Atom Has What Oxidation Number.
From www.numerade.com
SOLVED (a) Determine the formal charge on the chlorine atom in the Chlorine Atom Has What Oxidation Number what is the oxidation number for chlorine? oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. Electron configuration [na]3s 2 3p 5: The oxidation number for chlorine can vary depending on the compound it is. Allotropes some elements exist in several different structural forms, called allotropes. . Chlorine Atom Has What Oxidation Number.
From www.onlinechemistrytutor.net
An introduction to oxidation state Online Chemistry Tutor Chlorine Atom Has What Oxidation Number oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. The oxidation number for chlorine can vary depending on the compound it is. what is the oxidation number for chlorine? Allotropes some elements exist in several different structural forms, called allotropes. The oxidation number of an atom is. Chlorine Atom Has What Oxidation Number.
From linatusims.blogspot.com
Oxidation Number of Chlorine LinatuSims Chlorine Atom Has What Oxidation Number what is the oxidation number for chlorine? Electron configuration [na]3s 2 3p 5: oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. The oxidation number of an atom is defined as the charge that an atom. the oxidation state of an atom is equal to the. Chlorine Atom Has What Oxidation Number.
From study.com
Assigning Oxidation Numbers to Elements in a Chemical Formula Video Chlorine Atom Has What Oxidation Number The oxidation number for chlorine can vary depending on the compound it is. what is the oxidation number? what is the oxidation number for chlorine? Electron configuration [na]3s 2 3p 5: oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. The oxidation number of an atom. Chlorine Atom Has What Oxidation Number.
From www.sarthaks.com
When `Cl_(2)` gas reacts with hot and concentrated sodium hydroxide Chlorine Atom Has What Oxidation Number what is the oxidation number? The oxidation number of an atom is defined as the charge that an atom. Electron configuration [na]3s 2 3p 5: oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. what is the oxidation number for chlorine? the oxidation state of. Chlorine Atom Has What Oxidation Number.
From brainly.in
two oxidation state for chlorine are found in the compound (a) caocl2 Chlorine Atom Has What Oxidation Number The oxidation number of an atom is defined as the charge that an atom. oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. what is the oxidation number? the oxidation state of an atom is equal to the total number of electrons which have been removed. Chlorine Atom Has What Oxidation Number.
From www.sarthaks.com
When `Cl_(2)` gas reacts with hot and concentrated sodium hydroxide Chlorine Atom Has What Oxidation Number oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. what is the oxidation number for chlorine? the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. The oxidation number for chlorine can vary. Chlorine Atom Has What Oxidation Number.
From quizconsectary.z21.web.core.windows.net
Chlorine How Many Protons Electrons Neutrons Chlorine Atom Has What Oxidation Number what is the oxidation number for chlorine? Electron configuration [na]3s 2 3p 5: what is the oxidation number? Allotropes some elements exist in several different structural forms, called allotropes. oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. the oxidation state of an atom is. Chlorine Atom Has What Oxidation Number.
From askfilo.com
In which compound chlorine has highest oxidation number? Filo Chlorine Atom Has What Oxidation Number the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. The oxidation number of an atom is defined as the charge that an atom. what is the oxidation number for chlorine? what is the oxidation number? Electron configuration [na]3s 2 3p 5: Allotropes some. Chlorine Atom Has What Oxidation Number.
From sciencenotes.org
How to Assign Oxidation Numbers Chlorine Atom Has What Oxidation Number what is the oxidation number? Allotropes some elements exist in several different structural forms, called allotropes. Electron configuration [na]3s 2 3p 5: The oxidation number of an atom is defined as the charge that an atom. The oxidation number for chlorine can vary depending on the compound it is. oxidation state shows the total number of electrons which. Chlorine Atom Has What Oxidation Number.
From askfilo.com
The oxidation state of chlorine atom in hypochlorous acid is Only One Cor.. Chlorine Atom Has What Oxidation Number the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. what is the oxidation number for chlorine? what is the oxidation number? Electron configuration [na]3s 2 3p 5: The oxidation number for chlorine can vary depending on the compound it is. oxidation state. Chlorine Atom Has What Oxidation Number.
From www.vrogue.co
How Many Electrons And Protons Does An Atom Have 2023 vrogue.co Chlorine Atom Has What Oxidation Number The oxidation number for chlorine can vary depending on the compound it is. oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or. Electron configuration [na]3s 2 3p 5: what is the oxidation number for chlorine? what is the oxidation number? Allotropes some elements exist in several. Chlorine Atom Has What Oxidation Number.
From mungfali.com
Periodic Table Oxidation Chart Chlorine Atom Has What Oxidation Number the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. what is the oxidation number for chlorine? Allotropes some elements exist in several different structural forms, called allotropes. The oxidation number of an atom is defined as the charge that an atom. what is. Chlorine Atom Has What Oxidation Number.
From owlcation.com
AS Chemistry Redox Reactions and Group 2 Elements Owlcation Chlorine Atom Has What Oxidation Number Electron configuration [na]3s 2 3p 5: The oxidation number for chlorine can vary depending on the compound it is. The oxidation number of an atom is defined as the charge that an atom. Allotropes some elements exist in several different structural forms, called allotropes. what is the oxidation number for chlorine? the oxidation state of an atom is. Chlorine Atom Has What Oxidation Number.
From www.chemistrylearner.com
Oxidation Number (State) Definition, Rules, How to Find, and Examples Chlorine Atom Has What Oxidation Number the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. what is the oxidation number for chlorine? Allotropes some elements exist in several different structural forms, called allotropes. what is the oxidation number? The oxidation number for chlorine can vary depending on the compound. Chlorine Atom Has What Oxidation Number.
From www.scribd.com
Common Oxidation Numbers Chart Chlorine Atom Has What Oxidation Number what is the oxidation number for chlorine? Allotropes some elements exist in several different structural forms, called allotropes. the oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. Electron configuration [na]3s 2 3p 5: oxidation state shows the total number of electrons which have. Chlorine Atom Has What Oxidation Number.
From momzik.weebly.com
Periodic table chemistry with oxidation numbers momzik Chlorine Atom Has What Oxidation Number what is the oxidation number? The oxidation number of an atom is defined as the charge that an atom. Allotropes some elements exist in several different structural forms, called allotropes. what is the oxidation number for chlorine? oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or.. Chlorine Atom Has What Oxidation Number.
From www.nagwa.com
Question Video Determining the Oxidation Number of Atoms in a Molecule Chlorine Atom Has What Oxidation Number Electron configuration [na]3s 2 3p 5: The oxidation number for chlorine can vary depending on the compound it is. what is the oxidation number? The oxidation number of an atom is defined as the charge that an atom. Allotropes some elements exist in several different structural forms, called allotropes. oxidation state shows the total number of electrons which. Chlorine Atom Has What Oxidation Number.