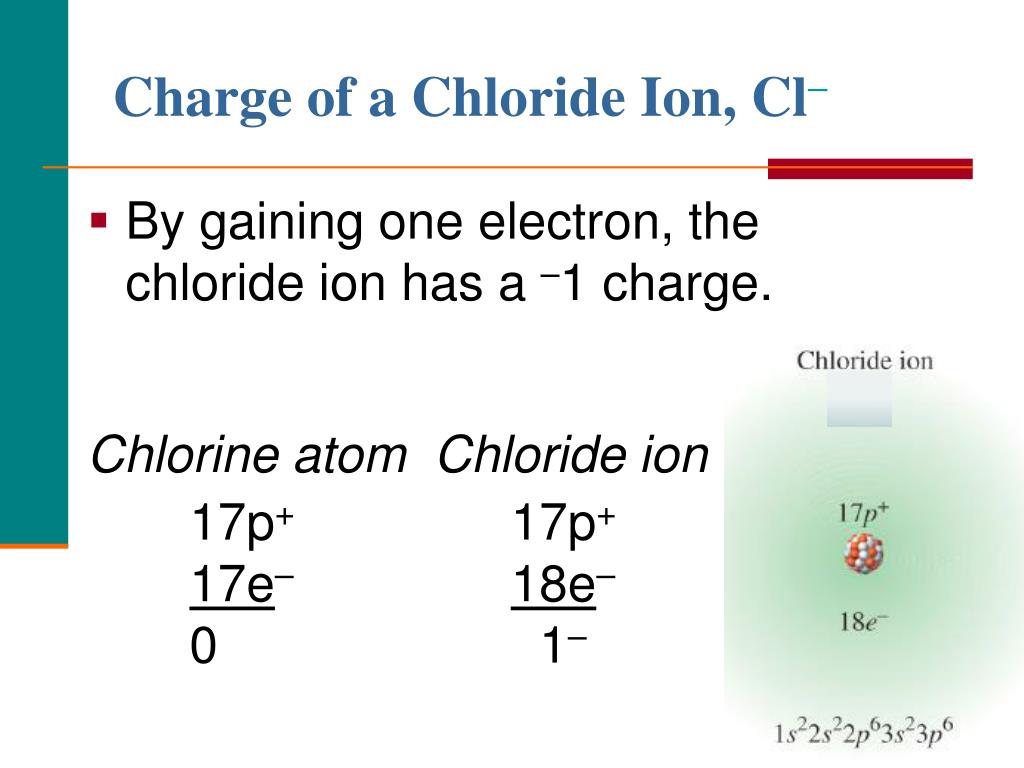
Chloride Ion And Valency . The valency of an ion is the number of electrical charges that it carries. Use this for reference with. The number of bonds that an atom can form as part of a compound is. Two h atoms combine with one o atom in h 2 o so do. The combining capacity, or valence, of o is apparently twice that of h or cl. Relative charge is called 'charge number' and given the symbol z. 3.3 valency and predicting formulas in ionic compounds. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. In ionic compounds, valency gives an indication of the charge an ion formed from a.
from www.slideserve.com
3.3 valency and predicting formulas in ionic compounds. Use this for reference with. The combining capacity, or valence, of o is apparently twice that of h or cl. Relative charge is called 'charge number' and given the symbol z. The number of bonds that an atom can form as part of a compound is. The valency of an ion is the number of electrical charges that it carries. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. In ionic compounds, valency gives an indication of the charge an ion formed from a. Two h atoms combine with one o atom in h 2 o so do.
PPT The Octet Rule PowerPoint Presentation, free download ID2654700
Chloride Ion And Valency In ionic compounds, valency gives an indication of the charge an ion formed from a. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Use this for reference with. In ionic compounds, valency gives an indication of the charge an ion formed from a. The valency of an ion is the number of electrical charges that it carries. The combining capacity, or valence, of o is apparently twice that of h or cl. The number of bonds that an atom can form as part of a compound is. Two h atoms combine with one o atom in h 2 o so do. 3.3 valency and predicting formulas in ionic compounds. Relative charge is called 'charge number' and given the symbol z.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chloride Ion And Valency The valency of an ion is the number of electrical charges that it carries. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Relative charge is called 'charge number' and given the symbol z. The number of bonds that an atom can form as part of a compound is. In ionic. Chloride Ion And Valency.
From collegedunia.com
The valency of barium in barium chloride is Chloride Ion And Valency Relative charge is called 'charge number' and given the symbol z. Use this for reference with. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. 3.3 valency and predicting formulas in ionic compounds. The valency of an ion is the number of electrical charges that it carries. In ionic compounds, valency. Chloride Ion And Valency.
From www.animalia-life.club
Sodium Chloride Lewis Dot Structure Chloride Ion And Valency Use this for reference with. Two h atoms combine with one o atom in h 2 o so do. In ionic compounds, valency gives an indication of the charge an ion formed from a. The number of bonds that an atom can form as part of a compound is. 3.3 valency and predicting formulas in ionic compounds. The valency of. Chloride Ion And Valency.
From www.doubtnut.com
Atoms and Molecules NCERT Class 9 Science Chloride Ion And Valency Relative charge is called 'charge number' and given the symbol z. The combining capacity, or valence, of o is apparently twice that of h or cl. The number of bonds that an atom can form as part of a compound is. 3.3 valency and predicting formulas in ionic compounds. Use this for reference with. Two h atoms combine with one. Chloride Ion And Valency.
From www.teachmint.com
Valency Table Science Notes Teachmint Chloride Ion And Valency Use this for reference with. The number of bonds that an atom can form as part of a compound is. Relative charge is called 'charge number' and given the symbol z. The combining capacity, or valence, of o is apparently twice that of h or cl. 93 rows this table of element valences includes the maximum valence and most common. Chloride Ion And Valency.
From guidelistpstrapanning.z14.web.core.windows.net
Diagram Ionic Bond Chloride Ion And Valency Relative charge is called 'charge number' and given the symbol z. In ionic compounds, valency gives an indication of the charge an ion formed from a. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. 3.3 valency and predicting formulas in ionic compounds. The valency of an ion is the number. Chloride Ion And Valency.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Chloride Ion And Valency Two h atoms combine with one o atom in h 2 o so do. Relative charge is called 'charge number' and given the symbol z. In ionic compounds, valency gives an indication of the charge an ion formed from a. The combining capacity, or valence, of o is apparently twice that of h or cl. The number of bonds that. Chloride Ion And Valency.
From www.wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Chloride Ion And Valency The combining capacity, or valence, of o is apparently twice that of h or cl. Use this for reference with. Relative charge is called 'charge number' and given the symbol z. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. The valency of an ion is the number of electrical charges. Chloride Ion And Valency.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chloride Ion And Valency The combining capacity, or valence, of o is apparently twice that of h or cl. In ionic compounds, valency gives an indication of the charge an ion formed from a. The valency of an ion is the number of electrical charges that it carries. The number of bonds that an atom can form as part of a compound is. Use. Chloride Ion And Valency.
From slideplayer.com
Ionic and covalent bonding ppt download Chloride Ion And Valency The number of bonds that an atom can form as part of a compound is. Use this for reference with. The combining capacity, or valence, of o is apparently twice that of h or cl. In ionic compounds, valency gives an indication of the charge an ion formed from a. 93 rows this table of element valences includes the maximum. Chloride Ion And Valency.
From byjus.com
In the electron dot structure, the valence shell electrons are Chloride Ion And Valency 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Relative charge is called 'charge number' and given the symbol z. The number of bonds that an atom can form as part of a compound is. Two h atoms combine with one o atom in h 2 o so do. The valency. Chloride Ion And Valency.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chloride Ion And Valency In ionic compounds, valency gives an indication of the charge an ion formed from a. 3.3 valency and predicting formulas in ionic compounds. Two h atoms combine with one o atom in h 2 o so do. The combining capacity, or valence, of o is apparently twice that of h or cl. Relative charge is called 'charge number' and given. Chloride Ion And Valency.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chloride Ion And Valency The combining capacity, or valence, of o is apparently twice that of h or cl. The number of bonds that an atom can form as part of a compound is. The valency of an ion is the number of electrical charges that it carries. In ionic compounds, valency gives an indication of the charge an ion formed from a. 3.3. Chloride Ion And Valency.
From slideplayer.com
Types of Bonding. ppt download Chloride Ion And Valency The valency of an ion is the number of electrical charges that it carries. The number of bonds that an atom can form as part of a compound is. The combining capacity, or valence, of o is apparently twice that of h or cl. Two h atoms combine with one o atom in h 2 o so do. Relative charge. Chloride Ion And Valency.
From schematron.org
Lewis Dot Diagram For Sodium Chloride Wiring Diagram Pictures Chloride Ion And Valency The valency of an ion is the number of electrical charges that it carries. Relative charge is called 'charge number' and given the symbol z. Two h atoms combine with one o atom in h 2 o so do. 3.3 valency and predicting formulas in ionic compounds. The combining capacity, or valence, of o is apparently twice that of h. Chloride Ion And Valency.
From slideplayer.com
Chemical formulae of ionic compounds ppt download Chloride Ion And Valency The number of bonds that an atom can form as part of a compound is. Relative charge is called 'charge number' and given the symbol z. Use this for reference with. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. In ionic compounds, valency gives an indication of the charge an. Chloride Ion And Valency.
From pasuukantujuh.blogspot.com
Cl Dot Structure Ionic Bonding CK12 Foundation / To draw the lewis Chloride Ion And Valency The combining capacity, or valence, of o is apparently twice that of h or cl. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Relative charge is called 'charge number' and given the symbol z. The number of bonds that an atom can form as part of a compound is. 3.3. Chloride Ion And Valency.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Chloride Ion And Valency Relative charge is called 'charge number' and given the symbol z. The combining capacity, or valence, of o is apparently twice that of h or cl. Two h atoms combine with one o atom in h 2 o so do. 3.3 valency and predicting formulas in ionic compounds. Use this for reference with. 93 rows this table of element valences. Chloride Ion And Valency.
From slideplayer.com
CHEMISTRY December 16, 2014 CHEMICAL BONDS. ppt download Chloride Ion And Valency Two h atoms combine with one o atom in h 2 o so do. The valency of an ion is the number of electrical charges that it carries. The combining capacity, or valence, of o is apparently twice that of h or cl. The number of bonds that an atom can form as part of a compound is. 3.3 valency. Chloride Ion And Valency.
From slideplayer.com
Ionic Bonding. ppt download Chloride Ion And Valency The number of bonds that an atom can form as part of a compound is. In ionic compounds, valency gives an indication of the charge an ion formed from a. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Two h atoms combine with one o atom in h 2 o. Chloride Ion And Valency.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chloride Ion And Valency The valency of an ion is the number of electrical charges that it carries. Use this for reference with. 3.3 valency and predicting formulas in ionic compounds. The number of bonds that an atom can form as part of a compound is. In ionic compounds, valency gives an indication of the charge an ion formed from a. Two h atoms. Chloride Ion And Valency.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Chloride Ion And Valency Relative charge is called 'charge number' and given the symbol z. The valency of an ion is the number of electrical charges that it carries. Use this for reference with. The combining capacity, or valence, of o is apparently twice that of h or cl. Two h atoms combine with one o atom in h 2 o so do. 93. Chloride Ion And Valency.
From www.askiitians.com
Chlorine Study Material for IIT JEE askIITians Chloride Ion And Valency 3.3 valency and predicting formulas in ionic compounds. Two h atoms combine with one o atom in h 2 o so do. The number of bonds that an atom can form as part of a compound is. In ionic compounds, valency gives an indication of the charge an ion formed from a. The combining capacity, or valence, of o is. Chloride Ion And Valency.
From dxopaibhs.blob.core.windows.net
A Chlorine Atom Gains An Electron. What Is The Resulting Particle at Chloride Ion And Valency The valency of an ion is the number of electrical charges that it carries. Relative charge is called 'charge number' and given the symbol z. The number of bonds that an atom can form as part of a compound is. Two h atoms combine with one o atom in h 2 o so do. The combining capacity, or valence, of. Chloride Ion And Valency.
From loeylrncp.blob.core.windows.net
Chlorine Electrons And Protons at Edward Thompson blog Chloride Ion And Valency The combining capacity, or valence, of o is apparently twice that of h or cl. Two h atoms combine with one o atom in h 2 o so do. In ionic compounds, valency gives an indication of the charge an ion formed from a. The valency of an ion is the number of electrical charges that it carries. Relative charge. Chloride Ion And Valency.
From www.youtube.com
Chlorine Electron Configuration YouTube Chloride Ion And Valency 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. 3.3 valency and predicting formulas in ionic compounds. Two h atoms combine with one o atom in h 2 o so do. Relative charge is called 'charge number' and given the symbol z. The number of bonds that an atom can form. Chloride Ion And Valency.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chloride Ion And Valency In ionic compounds, valency gives an indication of the charge an ion formed from a. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. 3.3 valency and predicting formulas in ionic compounds. The valency of an ion is the number of electrical charges that it carries. Two h atoms combine with. Chloride Ion And Valency.
From slideplayer.com
Mendeleev arranged the table by properties and then atomic mass. ppt Chloride Ion And Valency The combining capacity, or valence, of o is apparently twice that of h or cl. In ionic compounds, valency gives an indication of the charge an ion formed from a. The number of bonds that an atom can form as part of a compound is. Relative charge is called 'charge number' and given the symbol z. 3.3 valency and predicting. Chloride Ion And Valency.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Chloride Ion And Valency 3.3 valency and predicting formulas in ionic compounds. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. The valency of an ion is the number of electrical charges that it carries. The number of bonds that an atom can form as part of a compound is. In ionic compounds, valency gives. Chloride Ion And Valency.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chloride Ion And Valency The number of bonds that an atom can form as part of a compound is. Relative charge is called 'charge number' and given the symbol z. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. 3.3 valency and predicting formulas in ionic compounds. Two h atoms combine with one o atom. Chloride Ion And Valency.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chloride Ion And Valency The valency of an ion is the number of electrical charges that it carries. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Relative charge is called 'charge number' and given the symbol z. The combining capacity, or valence, of o is apparently twice that of h or cl. Use this. Chloride Ion And Valency.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chloride Ion And Valency The number of bonds that an atom can form as part of a compound is. The combining capacity, or valence, of o is apparently twice that of h or cl. Relative charge is called 'charge number' and given the symbol z. Two h atoms combine with one o atom in h 2 o so do. Use this for reference with.. Chloride Ion And Valency.
From astonishingceiyrs.blogspot.com
Cl Valence Electrons astonishingceiyrs Chloride Ion And Valency Use this for reference with. In ionic compounds, valency gives an indication of the charge an ion formed from a. The number of bonds that an atom can form as part of a compound is. 3.3 valency and predicting formulas in ionic compounds. The combining capacity, or valence, of o is apparently twice that of h or cl. The valency. Chloride Ion And Valency.
From byjus.com
What is valency of ions. Find the valency of ions of so3 Io2? Chloride Ion And Valency The valency of an ion is the number of electrical charges that it carries. The number of bonds that an atom can form as part of a compound is. The combining capacity, or valence, of o is apparently twice that of h or cl. Two h atoms combine with one o atom in h 2 o so do. 3.3 valency. Chloride Ion And Valency.
From chem.libretexts.org
4.3 Lewis Symbols and Structures Chemistry LibreTexts Chloride Ion And Valency Relative charge is called 'charge number' and given the symbol z. In ionic compounds, valency gives an indication of the charge an ion formed from a. The combining capacity, or valence, of o is apparently twice that of h or cl. 3.3 valency and predicting formulas in ionic compounds. Use this for reference with. The number of bonds that an. Chloride Ion And Valency.