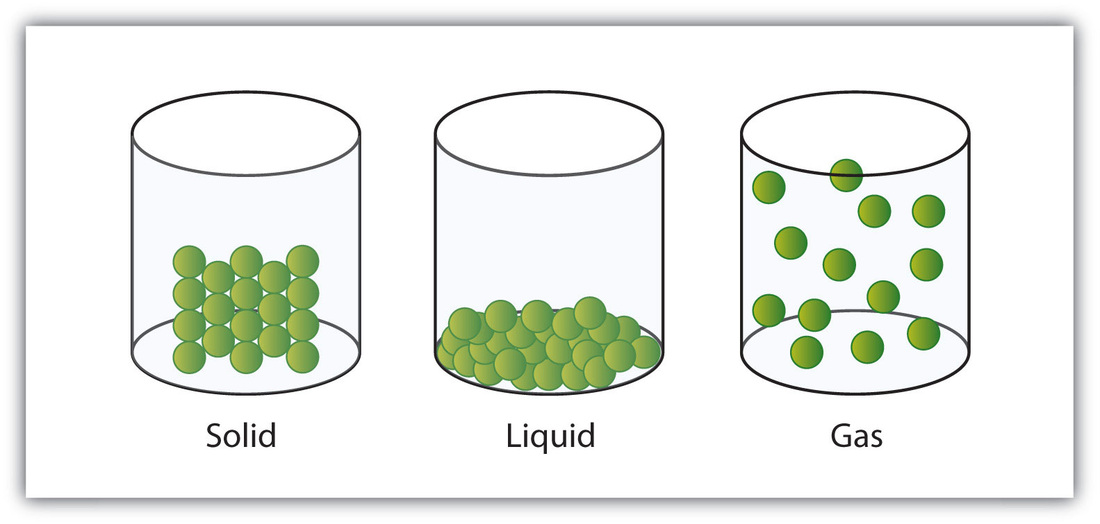
Solid Liquid Gas Line Graph . The blue line shows the boiling point, or transition between liquid and gaseous state, the green line shows the freezing point, or transition between liquid and solid state, and the red line shows the conditions where a solid state can be converted directly to gaseous phase and vice versa. Solid, liquid, gas, and a supercritical fluid. The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). Matter must be ionized to become plasma. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). The state exhibited by a given sample of matter depends on the identity, temperature, and pressure of the sample. The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. You can use a phase diagram to predict whether a substance will be a solid, liquid, or gas at a given combination of temperature and pressure. So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum. To be able to identify the triple point, the critical point, and four regions: Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). The triple point is the one condition of.
from joisrkcqc.blob.core.windows.net
A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). The blue line shows the boiling point, or transition between liquid and gaseous state, the green line shows the freezing point, or transition between liquid and solid state, and the red line shows the conditions where a solid state can be converted directly to gaseous phase and vice versa. The state exhibited by a given sample of matter depends on the identity, temperature, and pressure of the sample. Solid, liquid, gas, and a supercritical fluid. The triple point is the one condition of. Matter must be ionized to become plasma.
Which Properties Do All Solids Liquids And Gases Have at Estelle Lewis blog
Solid Liquid Gas Line Graph The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Solid, liquid, gas, and a supercritical fluid. To be able to identify the triple point, the critical point, and four regions: Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). The triple point is the one condition of. The blue line shows the boiling point, or transition between liquid and gaseous state, the green line shows the freezing point, or transition between liquid and solid state, and the red line shows the conditions where a solid state can be converted directly to gaseous phase and vice versa. So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. Matter must be ionized to become plasma. You can use a phase diagram to predict whether a substance will be a solid, liquid, or gas at a given combination of temperature and pressure. The state exhibited by a given sample of matter depends on the identity, temperature, and pressure of the sample.
From joisrkcqc.blob.core.windows.net
Which Properties Do All Solids Liquids And Gases Have at Estelle Lewis blog Solid Liquid Gas Line Graph The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas. Solid Liquid Gas Line Graph.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Solid Liquid Gas Line Graph So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. Matter must be ionized to become plasma. The green line divides the solid and liquid phases and. Solid Liquid Gas Line Graph.
From exyfeersx.blob.core.windows.net
Why Solid Liquid And Gas Have Different Properties at Helen Gatlin blog Solid Liquid Gas Line Graph Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum. To be able to identify the triple point, the critical point, and four regions: The following plot shows the gibbs energy as a function of temperature,. Solid Liquid Gas Line Graph.
From www.pinterest.ph
Properties of Solids, Liquids, Gases Compared Teachoo Science Solid Liquid Gas Line Graph Matter must be ionized to become plasma. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum. The triple point is. Solid Liquid Gas Line Graph.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Solid Liquid Gas Line Graph To be able to identify the triple point, the critical point, and four regions: Matter must be ionized to become plasma. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). The. Solid Liquid Gas Line Graph.
From classnotes.org.in
Solubility of Gases and Solids in Liquids Chemistry, Class 12, Solutions Solid Liquid Gas Line Graph The triple point is the one condition of. The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). Matter must be ionized to become plasma. Heat, cool. Solid Liquid Gas Line Graph.
From letsraceturtles.blogspot.com
Things Chapter 5 Gibbs Free Energy Solid Liquid Gas Line Graph A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). To be able to identify the triple point, the critical point, and four regions: The triple point is the one condition of. Matter must be ionized to. Solid Liquid Gas Line Graph.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Solid Liquid Gas Line Graph Matter must be ionized to become plasma. You can use a phase diagram to predict whether a substance will be a solid, liquid, or gas at a given combination of temperature and pressure. The triple point is the one condition of. So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go. Solid Liquid Gas Line Graph.
From samson-jolpblogsantos.blogspot.com
How Does Temperature Affect Solids Liquids and Gases Solid Liquid Gas Line Graph Solid, liquid, gas, and a supercritical fluid. So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). Heat, cool and compress atoms and molecules and. Solid Liquid Gas Line Graph.
From byjus.com
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law Solid Liquid Gas Line Graph So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum. The blue line shows the boiling point, or transition between liquid and gaseous state, the green line shows the freezing point, or transition between liquid and solid state, and the red line shows the conditions. Solid Liquid Gas Line Graph.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book Solid Liquid Gas Line Graph Matter must be ionized to become plasma. Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. The triple point is the one condition of. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists. Solid Liquid Gas Line Graph.
From www.iitianacademy.com
AP Chemistry Unit 3.3 Solids, Liquids, and Gas Solid Liquid Gas Line Graph The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). The blue line shows the boiling point, or transition between liquid and gaseous state, the green line shows the freezing point, or transition between liquid and solid state, and the red line shows the conditions where a solid state can. Solid Liquid Gas Line Graph.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Solid Liquid Gas Line Graph Solid, liquid, gas, and a supercritical fluid. Matter must be ionized to become plasma. The triple point is the one condition of. The state exhibited by a given sample of matter depends on the identity, temperature, and pressure of the sample. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to. Solid Liquid Gas Line Graph.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Solid Liquid Gas Line Graph So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum. To be able to identify the triple point, the critical point, and four regions: Solid, liquid, gas, and a supercritical fluid. Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). The following plot shows the. Solid Liquid Gas Line Graph.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid Liquid Gas Line Graph Solid, liquid, gas, and a supercritical fluid. The state exhibited by a given sample of matter depends on the identity, temperature, and pressure of the sample. Matter must be ionized to become plasma. So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum. To be. Solid Liquid Gas Line Graph.
From www.mikrora.com
Gas Liquid And Solid Diagram Solid Liquid Gas Line Graph The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). You can use a phase diagram to predict whether a substance will be a solid, liquid, or gas at a given combination of temperature and pressure.. Solid Liquid Gas Line Graph.
From courses.lumenlearning.com
Phase Diagrams Chemistry for Majors Solid Liquid Gas Line Graph Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). The triple point is the one condition of. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. Matter. Solid Liquid Gas Line Graph.
From studydbbelford.z5.web.core.windows.net
Science Solid Liquid Gas Solid Liquid Gas Line Graph The state exhibited by a given sample of matter depends on the identity, temperature, and pressure of the sample. Matter must be ionized to become plasma. To be able to identify the triple point, the critical point, and four regions: The blue line shows the boiling point, or transition between liquid and gaseous state, the green line shows the freezing. Solid Liquid Gas Line Graph.
From mungfali.com
Solids Liquids Gases Chart Solid Liquid Gas Line Graph The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). The blue line shows the boiling. Solid Liquid Gas Line Graph.
From mungfali.com
Solid Liquid Gas Phase Change Diagram Solid Liquid Gas Line Graph So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum. The blue line shows the boiling point, or transition between liquid and gaseous state, the green line shows the freezing point, or transition between liquid and solid state, and the red line shows the conditions. Solid Liquid Gas Line Graph.
From www.youtube.com
States of Matter Solid Liquid Gas States of Matter drawing Different Solid Liquid Gas Line Graph The triple point is the one condition of. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). The blue line shows the boiling point, or transition between liquid and gaseous state, the green line shows the. Solid Liquid Gas Line Graph.
From mungfali.com
Solids Liquids Gases Chart Solid Liquid Gas Line Graph Solid, liquid, gas, and a supercritical fluid. Matter must be ionized to become plasma. Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. To be able to identify the triple point, the critical point, and four regions: The green line divides the solid and liquid phases and represents melting (solid to. Solid Liquid Gas Line Graph.
From madanapandita.blogspot.com
Phase Diagram Thermodynamics Solid Liquid Gas Line Graph The triple point is the one condition of. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). Solid, liquid, gas, and a supercritical fluid. So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum.. Solid Liquid Gas Line Graph.
From www.rapidonline.com
Solids, Liquids, Gases Wall Chart Rapid Online Solid Liquid Gas Line Graph The blue line shows the boiling point, or transition between liquid and gaseous state, the green line shows the freezing point, or transition between liquid and solid state, and the red line shows the conditions where a solid state can be converted directly to gaseous phase and vice versa. Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). Solid, liquid,. Solid Liquid Gas Line Graph.
From courses.lumenlearning.com
Phase Diagrams Chemistry for Majors Solid Liquid Gas Line Graph The triple point is the one condition of. Solid, liquid, gas, and a supercritical fluid. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas. Solid Liquid Gas Line Graph.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Solid Liquid Gas Line Graph Matter must be ionized to become plasma. The blue line shows the boiling point, or transition between liquid and gaseous state, the green line shows the freezing point, or transition between liquid and solid state, and the red line shows the conditions where a solid state can be converted directly to gaseous phase and vice versa. The triple point is. Solid Liquid Gas Line Graph.
From chemistry.stackexchange.com
gas laws Pressure Vs Volume plot for real and ideal gasses Solid Liquid Gas Line Graph You can use a phase diagram to predict whether a substance will be a solid, liquid, or gas at a given combination of temperature and pressure. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. The blue line shows the boiling point, or transition between. Solid Liquid Gas Line Graph.
From www.studypool.com
SOLUTION Pearson igcse physics notes 5 solids liquids gases heating Solid Liquid Gas Line Graph Matter must be ionized to become plasma. The triple point is the one condition of. The blue line shows the boiling point, or transition between liquid and gaseous state, the green line shows the freezing point, or transition between liquid and solid state, and the red line shows the conditions where a solid state can be converted directly to gaseous. Solid Liquid Gas Line Graph.
From unistudium.unipg.it
Phase Diagrams Solid Liquid Gas Line Graph The triple point is the one condition of. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum. To be able to identify the triple. Solid Liquid Gas Line Graph.
From socratic.org
How do graph temperature versus time for a pure substance? Socratic Solid Liquid Gas Line Graph The triple point is the one condition of. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). Matter must be ionized to become plasma. Solid, liquid, gas, and a supercritical fluid. The blue line shows the. Solid Liquid Gas Line Graph.
From guidelistbaquantising.z13.web.core.windows.net
Venn Diagram For Solids Liquids And Gases Solid Liquid Gas Line Graph The blue line shows the boiling point, or transition between liquid and gaseous state, the green line shows the freezing point, or transition between liquid and solid state, and the red line shows the conditions where a solid state can be converted directly to gaseous phase and vice versa. Heat, cool and compress atoms and molecules and watch as they. Solid Liquid Gas Line Graph.
From www.etsy.com
Science, States of Matter, Solid, Liquid, Gas, Elementary, Anchor Chart Solid Liquid Gas Line Graph The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). Solid, liquid, gas, and a supercritical fluid. The triple point is the one condition of. So, you can increase temperature to form ions, but decreasing pressure doesn’t automatically make plasma even if you go all the way to a vacuum.. Solid Liquid Gas Line Graph.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws Solid Liquid Gas Line Graph Matter must be ionized to become plasma. The following plot shows the gibbs energy as a function of temperature, including phase changes from solid to liquid (melting) and liquid to gas (boiling). Solid, liquid, gas, and a supercritical fluid. Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). To be able to identify the triple point, the critical point, and. Solid Liquid Gas Line Graph.
From shop.omegascientific.com.au
CHART, Solids, Liquids and Gases Solid Liquid Gas Line Graph Matter must be ionized to become plasma. The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. You can. Solid Liquid Gas Line Graph.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Solid Liquid Gas Line Graph The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). Gibbs energy (\(\bar{g}\)) as a function of temperature (\(t\)). The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). The following plot shows the gibbs energy as a function of temperature, including. Solid Liquid Gas Line Graph.