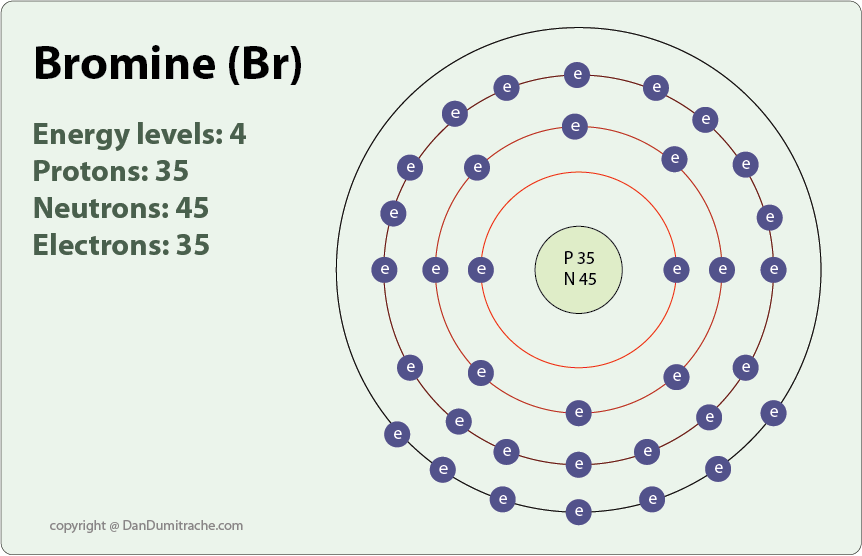
Bromine Fluorine Group . The halogen elements are fluorine (f),. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Includes trends in atomic and. — covers the halogens in group 17: — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). The fall in reactivity is because the halogens become less.
from quizdbpharmacies.z4.web.core.windows.net
chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — covers the halogens in group 17: — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Includes trends in atomic and. The halogen elements are fluorine (f),. The fall in reactivity is because the halogens become less.
Number Of Protons And Neutrons In Fluorine
Bromine Fluorine Group Includes trends in atomic and. Includes trends in atomic and. The halogen elements are fluorine (f),. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. — covers the halogens in group 17: Fluorine (f), chlorine (cl), bromine (br) and iodine (i). The fall in reactivity is because the halogens become less.
From www.youtube.com
BrF3 Lewis Structure (Bromine Trifluoride) YouTube Bromine Fluorine Group The halogen elements are fluorine (f),. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. The fall in reactivity is because the halogens become less. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). —. Bromine Fluorine Group.
From www.dreamstime.com
Bromine fluoride molecule stock illustration. Illustration of bonding Bromine Fluorine Group — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. — covers the halogens in group 17: Fluorine (f), chlorine (cl), bromine (br) and iodine (i). chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. The halogen elements are fluorine (f),. Includes trends in atomic. Bromine Fluorine Group.
From slidetodoc.com
HALOGENS Chlorine Fluorine GROUP 7 A Bromine Iodine Bromine Fluorine Group — covers the halogens in group 17: chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Includes trends in atomic and. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). The halogen elements are fluorine (f),. The fall in reactivity is because the halogens become less. — halogen, any of the six nonmetallic. Bromine Fluorine Group.
From slideplayer.com
THE HALOGENS. ppt download Bromine Fluorine Group — covers the halogens in group 17: chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The halogen elements are fluorine (f),. Includes trends in atomic and. Fluorine (f), chlorine (cl), bromine (br) and iodine. Bromine Fluorine Group.
From www.alamy.com
Bromine symbol. Chemical element of the periodic table. Vector stock Bromine Fluorine Group Includes trends in atomic and. The fall in reactivity is because the halogens become less. — covers the halogens in group 17: — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). The halogen elements are fluorine (f),. chlorine and. Bromine Fluorine Group.
From animalia-life.club
Fluorine Atom 3d Model Bromine Fluorine Group Includes trends in atomic and. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — covers the halogens in group 17: The halogen elements are fluorine (f),. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic. Bromine Fluorine Group.
From slideplayer.com
IPS Unit 8 Periodic Table Section ppt download Bromine Fluorine Group Includes trends in atomic and. — covers the halogens in group 17: — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The halogen elements are fluorine (f),. The fall in reactivity is because the halogens become less. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). chlorine and. Bromine Fluorine Group.
From dxoqxvxev.blob.core.windows.net
How Many Valence Electrons Does Bromine Want at Jan Myers blog Bromine Fluorine Group Fluorine (f), chlorine (cl), bromine (br) and iodine (i). — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Includes trends in atomic and. The fall in reactivity is because the halogens become less. — covers the halogens in group 17: The halogen elements are fluorine (f),. chlorine and. Bromine Fluorine Group.
From www.shutterstock.com
3d Render Bromine Fluoride Stock Illustration 26187403 Shutterstock Bromine Fluorine Group — covers the halogens in group 17: chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Includes trends in atomic and. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). The fall in reactivity is. Bromine Fluorine Group.
From stock.adobe.com
Halogens. 7A group of the periodic table. Vector illustration. Chemical Bromine Fluorine Group — covers the halogens in group 17: Fluorine (f), chlorine (cl), bromine (br) and iodine (i). The halogen elements are fluorine (f),. The fall in reactivity is because the halogens become less. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. chlorine and bromine produce iron(iii) chloride or. Bromine Fluorine Group.
From www.dreamstime.com
Bromine Chemical 35 Element of Periodic Table. Molecule and Bromine Fluorine Group chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. The halogen elements are fluorine (f),. — covers the halogens in group 17: Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Includes trends in atomic and. The fall in reactivity is because the halogens become less. — halogen, any of the six nonmetallic. Bromine Fluorine Group.
From www.youtube.com
How to draw Lewis dot structures Fluorine(F) Chlorine(Cl) Bromine(Br Bromine Fluorine Group Fluorine (f), chlorine (cl), bromine (br) and iodine (i). — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. — covers the halogens in group 17: The halogen elements are fluorine (f),. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Includes trends in atomic. Bromine Fluorine Group.
From www.youtube.com
Group 7 Elements. Halogens. Salt Formers. Fluorine, Chlorine, Bromine Bromine Fluorine Group — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — covers the halogens in group 17: Includes trends in atomic and. The fall in reactivity is because the halogens become less. Fluorine (f), chlorine (cl),. Bromine Fluorine Group.
From dxowxzkca.blob.core.windows.net
Fluorine Chlorine Bromine at Alphonse Sparks blog Bromine Fluorine Group The halogen elements are fluorine (f),. The fall in reactivity is because the halogens become less. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Includes trends. Bromine Fluorine Group.
From dxowxzkca.blob.core.windows.net
Fluorine Chlorine Bromine at Alphonse Sparks blog Bromine Fluorine Group — covers the halogens in group 17: The halogen elements are fluorine (f),. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). The fall in reactivity is because the halogens become less. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. chlorine and bromine produce iron(iii) chloride or. Bromine Fluorine Group.
From medium.com
What is fluorine?. Fluorine in the periodic table by Chemistry Topics Bromine Fluorine Group Fluorine (f), chlorine (cl), bromine (br) and iodine (i). chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — covers the halogens in group 17: The halogen elements are fluorine (f),. The fall in reactivity is because the halogens become less. Includes trends in atomic and. — halogen, any of the six nonmetallic. Bromine Fluorine Group.
From slidetodoc.com
The periodic table Describe the chemical reactions that Bromine Fluorine Group The halogen elements are fluorine (f),. — covers the halogens in group 17: — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Includes trends in atomic and. The fall in reactivity is because the halogens. Bromine Fluorine Group.
From animalia-life.club
Element Fluorine Gas Bromine Fluorine Group The fall in reactivity is because the halogens become less. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. The halogen elements are fluorine (f),. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. — covers the halogens in group 17: Includes trends in. Bromine Fluorine Group.
From guidefixbukukurj.z21.web.core.windows.net
Bohr Model Of Fluorine Atom Bromine Fluorine Group — covers the halogens in group 17: The fall in reactivity is because the halogens become less. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The halogen elements are fluorine (f),. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). chlorine and bromine produce iron(iii) chloride or. Bromine Fluorine Group.
From www.alamy.com
Bromine. Bromum. Halogens. Chemical Element of Mendeleev's Periodic Bromine Fluorine Group The halogen elements are fluorine (f),. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — covers the halogens in group 17: The fall in reactivity is because the halogens become less. Includes trends in atomic and. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). — halogen, any of the six nonmetallic. Bromine Fluorine Group.
From chemisfast.blogspot.com
Halogen family elementspropertiesperiodic tableoxyacids Bromine Fluorine Group — covers the halogens in group 17: — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Includes trends in atomic and. The halogen elements are fluorine (f),. The fall in reactivity is because the halogens. Bromine Fluorine Group.
From app.pandai.org
Elements in Group 17 Bromine Fluorine Group Fluorine (f), chlorine (cl), bromine (br) and iodine (i). The fall in reactivity is because the halogens become less. Includes trends in atomic and. The halogen elements are fluorine (f),. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of. Bromine Fluorine Group.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Bromine Fluorine Group Fluorine (f), chlorine (cl), bromine (br) and iodine (i). The halogen elements are fluorine (f),. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Includes trends in atomic and. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. The fall in reactivity is because the. Bromine Fluorine Group.
From exobvmtcw.blob.core.windows.net
Bromine Fluoride Structure at Guadalupe Lewis blog Bromine Fluorine Group — covers the halogens in group 17: — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The halogen elements are fluorine (f),. Includes trends in atomic and. The fall in reactivity is because the halogens become less. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces. Bromine Fluorine Group.
From dxowxzkca.blob.core.windows.net
Fluorine Chlorine Bromine at Alphonse Sparks blog Bromine Fluorine Group — covers the halogens in group 17: chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The halogen elements are fluorine (f),. Includes trends in atomic. Bromine Fluorine Group.
From www.alamy.com
halogens in gas jars Fl Cl Br I fluorine chlorine bromine iodine Stock Bromine Fluorine Group — covers the halogens in group 17: The fall in reactivity is because the halogens become less. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). The halogen elements are fluorine (f),. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — halogen, any of the six nonmetallic elements that constitute group 17. Bromine Fluorine Group.
From nap.nationalacademies.org
Table of Isotopes of Fluorine, Chlorine, Bromine and Iodine The Bromine Fluorine Group — covers the halogens in group 17: — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The halogen elements are fluorine (f),. Includes trends in atomic and. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii). Bromine Fluorine Group.
From quizdbpharmacies.z4.web.core.windows.net
Number Of Protons And Neutrons In Fluorine Bromine Fluorine Group Fluorine (f), chlorine (cl), bromine (br) and iodine (i). — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. — covers the halogens in group 17: The fall in reactivity is because the halogens become less. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide.. Bromine Fluorine Group.
From bromineinformation.weebly.com
Bromine on the Periodic Table Bromine Fluorine Group The halogen elements are fluorine (f),. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. The fall in reactivity is because the halogens become less. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Includes trends. Bromine Fluorine Group.
From dxoqxvxev.blob.core.windows.net
How Many Valence Electrons Does Bromine Want at Jan Myers blog Bromine Fluorine Group Includes trends in atomic and. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — covers the halogens in group 17: The halogen elements are fluorine (f),. Fluorine (f), chlorine (cl), bromine (br) and iodine. Bromine Fluorine Group.
From www.numerade.com
Bromine is a halogen; one of the elements in the same column of the Bromine Fluorine Group The halogen elements are fluorine (f),. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Includes trends in atomic and. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. — covers the halogens in group 17: The fall in reactivity is because the halogens become less. chlorine and. Bromine Fluorine Group.
From www.alamy.com
Fluorine, Bromine and Chlorine Molecular Model of Atom. Vector Bromine Fluorine Group chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). The fall in reactivity is because the halogens become less. Includes trends in atomic and. The halogen elements. Bromine Fluorine Group.
From www.studypool.com
SOLUTION Aga Khan High school Mombasa chemistry 233 Group 7 elements Bromine Fluorine Group The halogen elements are fluorine (f),. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — covers the halogens in group 17: Includes trends in atomic and. The fall in reactivity is because the halogens become less. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). — halogen, any of the six nonmetallic. Bromine Fluorine Group.
From cabinet.matttroy.net
Fluorine Periodic Table Group Matttroy Bromine Fluorine Group Includes trends in atomic and. The fall in reactivity is because the halogens become less. — covers the halogens in group 17: — halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The halogen elements are fluorine (f),. chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces. Bromine Fluorine Group.
From unacademy.com
What is the Hybridization of Bromine Pentafluoride Bromine Fluorine Group The halogen elements are fluorine (f),. Includes trends in atomic and. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. — covers the halogens in group 17: The fall in reactivity is because the halogens become less. — halogen, any of the six nonmetallic. Bromine Fluorine Group.